Applicability of consensus methods in hip arthroscopy: a systematic review
Aplicabilidad de los métodos de consenso en la artroscopia de cadera: revisión sistemática
Resumen:
Objetivo: los métodos de consenso pueden convertirse en una herramienta útil cuando la evidencia disponible es escasa. El aumento de las indicaciones de la artroscopia de cadera hace necesario el uso de estudios con metodología de consenso. El objetivo de este estudio fue identificar los métodos de consenso empleados en el ámbito de la artroscopia de cadera.
Material y método: se realizó una revisión sistemática mediante consulta a las bases de datos MEDLINE y PreMEDLINE (Ovid), Embase, Scopus, Web Of Science y recursos de evaluación de tecnologías sanitarias, hasta septiembre de 2021. Se incluyeron estudios que emplearan la técnica Delphi, el método RAND/UCLA, el grupo nominal, las conferencias de consenso o discusiones informales, e implicaran a pacientes con cualquier patología de cadera sometidos a una artroscopia de cadera. Dos revisores llevaron a cabo la selección de los estudios de forma independiente. La extracción de los datos relevantes fue realizada por un revisor y comprobada por un segundo revisor. La evaluación de la calidad de realizó por pares y mediante una lista de comprobación.
Resultados: se seleccionaron 13 estudios, correspondientes a 10 artículos originales, 2 informes de evaluación de tecnologías sanitarias y 1 documento de criterios de uso apropiado. De los 13 estudios, 4 emplearon métodos no estructurados y 9 utilizaron métodos estructurados como la conferencia de consenso, la técnica Delphi, el grupo nominal y la metodología RAND/UCLA. Los estudios con métodos formales de consenso obtuvieron puntuaciones más elevadas en la evaluación de la calidad metodológica.
Conclusiones: los métodos de consenso que evaluaron la utilización de la artroscopia en patología de cadera fueron mayoritariamente métodos formales de consenso. La utilización de estos métodos estructurados permitió conseguir, en su mayoría, los criterios para establecer el consenso entre los profesionales.
Abstract:
Objective: consensus methods may represent a useful tool when the available evidence is scarce. The increase in the indications of hip arthroscopy makes it necessary to carry out studies with consensus methodology. The present study was carried out to identify the consensus methods used in the hip arthroscopy setting.
Material and methods: a systematic review was made consulting MEDLINE and PreMEDLINE (Ovid), Embase, Scopus, Web Of Science databases and healthcare technology evaluation resources up until September 2021. Inclusion was made of studies using the Delphi technique, the RAND/UCLA method, nominal group, consensus conferences or informal discussions, and which involved patients with hip disease of any kind subjected to hip arthroscopy. Two reviewers selected the studies on an independent basis. Relevant data were extracted by a reviewer and checked by a second reviewer. Quality assessment was performed on a paired basis and using a checklist.
Results: a total of 13 studies were selected, corresponding to 10 original articles, two healthcare technology evaluation reports and one appropriate use criteria document. Of the 13 studies, four used non-structured methods and 9 used structured methods such as the consensus conference, Delphi technique, nominal group and RAND/UCLA methodology. The studies involving formal consensus methods yielded higher methodological quality assessment scores.
Conclusions: the consensus methods evaluating the use of arthroscopy in hip disease were predominantly formal consensus methods. In most cases, the use of these structured methods provided the criteria needed to establish consensus among the professionals.
Introduction
In some cases, when answers to very concrete questions are needed, or when addressing relatively novel issues, the scientific evidence may be lacking, or the existing evidence may be of suboptimal quality. In such cases it is necessary to resort to the opinion and experience of experts, adopting a systematic approach based on consensus methodology, in order to explore the level of agreement or disagreement on a given subject. Thus, the need for consensus arises from the lack of consensus(1).
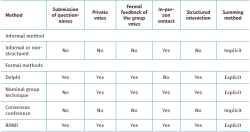
Among the different consensus methods, informal consensus is based on open and non-systematised discussion that often takes place in the context of a single physical meeting. It affords recommendations and very little information on the basis sustaining the consensus. Informal consensus is obviously very susceptible to bias and group effects(2,3), and since the anonymity of the participants is not guaranteed, some opinions are often not expressed or are diluted among those of other more reputed experts(4). In contrast, formal consensus combines scientific evidence with methodological techniques and structured processes in the making of decisions. In the healthcare setting, use fundamentally has been made of three formal consensus methods(5): the Delphi technique was introduced in the 1960s(6), followed by the nominal group technique in the 1970s(7) and, in 1977, by the consensus conference method developed by the United States National Institute of Health Consensus Development Program (8).
A fourth strategy, the RAND/UCLA method, was developed in the 1980s by the RAND Corporation and the University of California at Los Angeles (UCLA), and constitutes a hybrid of the Delphi and nominal group methods(9).
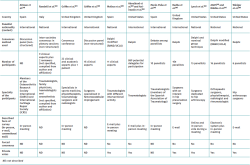
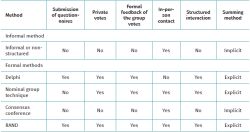
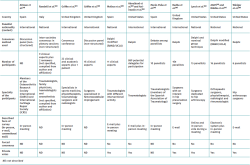
Table 1 highlights some of the differences between these approaches(5).
Although there are variations in the consensus methods, they all follow highly formalised protocols and share a number of fundamental principles that distinguish them from informal consensus: anonymity, iteration, controlled feedback, group statistical response and structured interaction(10,11).
Although these methods have recently been used in traumatology(12,13,14,15), it would be advisable to specifically examine their use in the context of hip arthroscopy. Based on the use of these consensus techniques, it has been attempted to synthesise the collective opinions in a current state of uncertainty (differences in opinion on different aspects of the approach to hip arthroscopy). For this reason, we raised the hypothesis that there are quality publications related to consensus methods in hip arthroscopy which could guide clinical decisions in the event of the lack of an adequate body of evidence.
The general objective of the present study was to identify the consensus methods used in the hip arthroscopy setting.
Material and methods
A systematic review was made of the literature using formal methods to ensure a pertinent and precise search and retrieval process. The present study was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement(16,17). Thus, before starting the literature search and subsequent data extraction, a review protocol was developed, describing each step of the systematic review, including the exclusion criteria. This protocol was reviewed and approved by three of the authors.
Information sources and search strategy
Systematic searches were made in September 2021 (without time restrictions) in the following electronic databases: MEDLINE and PreMEDLINE (Ovid), Embase, Scopus and Web Of Science (WOS), combining terms such as consensus, Delphi, RAND, nominal, hip or arthroscopy. A manual review was made of the references of all the selected articles in order to locate other studies not appearing in the first search. We consulted the Centre for Reviews and Dissemination (CRD) and International Network of Agencies for Health Technology Assessment (INAHTA) databases. In addition, we consulted the website of the Spanish Network of Agencies for the Evaluation of Healthcare Technologies and Services of the National Healthcare System (Red Española de Agencias de Evaluación de Tecnologías Sanitarias y Prestaciones del Sistema Nacional de Salud [RedETS]). An example of the MEDLINE search is provided in Figure 1. The rest of the literature searches are available upon request addressed to the corresponding author.
Selection of studies
On an independent and paired basis, two reviewers selected the studies by reading the titles and abstracts located through the scientific literature search. The full-text versions of the selected articles were reviewed and classified as included or excluded by the two reviewers, based on the established screening criteria. When doubts or discrepancies were found, they were resolved by consensus. The following study selection criteria were applied:
- Types of studies: consensuses using the Delphi technique, the RAND method, nominal group, consensus conferences or informal discussions.
- Participants: patients with hip disease of any kind.
- Intervention: any disease condition implying hip arthroscopy.
- Comparator: any.
- Outcome measures: data were extracted related to identification of the study, with the design and methodology.
Communications at congresses, letters to the editor, editorials and comments were excluded. We also excluded studies not written in Spanish, English, French, Portuguese or Italian.
Data extraction
Data extraction from the included studies was carried out by a reviewer and checked by a second reviewer. Doubts or discrepancies were resolved by consensus. The data were entered on customised electronic sheets.
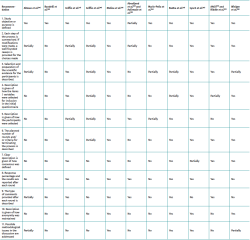
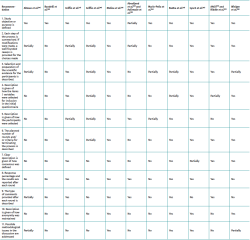
The data extraction procedure was carried out in two phases. The first phase compiled information referred to identification of the study, such as the year of conduction / publication of the study, country and organising entity. The second phase of the data extraction procedure included the consensus method used, the purpose of the study, the number of participants and the setting (national or international). In addition, we determined whether a literature review was made, whether the participants received prior information, whether the survey method was described (survey sent by conventional mail, e-mail or conducted on an in-person basis), the number of rounds and the number of participants that responded in each round, whether voting was private and whether anonymity was preserved, whether there was a predetermined definition of consensus and if so, what was the definition. Lastly, in the event of consensus, we evaluated whether it constituted forced consensus. The reviewers evaluated not only the presence or absence of each of these parameters in the studies but also whether they had been explicitly declared and described in sufficient detail.
Quality assessment
The recommendations of Humphrey-Murto et al.(18) were followed to guarantee methodological rigour in using the consensus group methods. These recommendations afford a checklist with the purpose of providing all the information that is essential for writing, interpreting and correctly using the results of a consensus. In general terms, the greater the compiled evidence in support of the development or choice of a method, the more reliable the findings derived from its use will be.
Data synthesis
A narrative synthesis was made, with tabulation of the information collected from the included studies.
Results
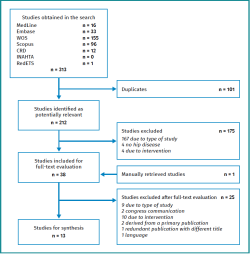
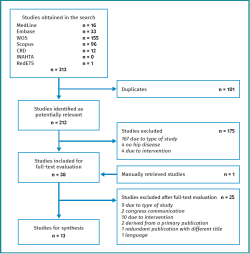
Figure 2 shows the study selection process. The search in the aforementioned electronic databases resulted in the identification of 313 literature references; this figure was reduced to 212 after removing duplicates. Following the selection process, we finally included 13 studies corresponding to 10 original papers(19,20,21,22,23,24,25,26,27,28), two healthcare technology evaluation reports(29,30) and one appropriate use criteria document adopted by the steering committee of the American Academy of Orthopaedic Surgeons (AAOS)(31).
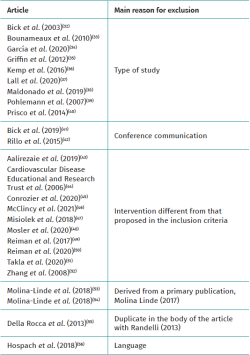
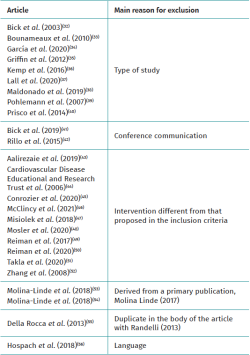
Table 2 shows the studies discarded after full-text evaluation(32-56) and the reasons for exclusion.
Of the 13 finally included studies, four used non-structured methods(19,20,24,30), while 9 used structured methods such as consensus conference(21), Delphi(22,23,25,28), Delphi together with nominal group(26) and RAND/UCLA methodology(27,29,31). The number of consulted specialists ranged between 9-869, and most of them were specialised in traumatology. The principal characteristics of the studies are described in Table 3.
Most of the studies addressed degenerative diseases such as osteoarthritis(19,27,29,31) or femoroacetabular impingement(21,24,25,26,28,29). Other addressed aspects were referred to hip dysplasia(24), infections(22,23), thromboembolism(20) and clinical trial screening criteria(30).
The study published by Altman et al.(19) was designed to describe the best available measurement method for detecting the progression of hip osteoarthritis, particularly in therapeutic trials. Consensus determined that radiography is an adequate primary assessment method for changes in hip osteoarthritis. The progression of osteoarthritis can be calculated by measuring the width of the joint space.
The report by Molina et al.(29) developed criteria for the appropriate or adequate use of hip arthroscopy in osteoarthritis and femoroacetabular impingement. Hip arthroscopy was considered generally inadequate as surgical treatment for osteoarthritis and adequate for femoroacetabular impingement, based on the presence of the following criteria: joint clinical manifestations, duration of the symptoms, functional alteration and patient age.
The AAOS consensus(31), also contemplated in the study of Riddle et al.(27), included pharmacological and non-pharmacological aspects, and surgical procedures, for symptomatic hip osteoarthritis. The suitability of hip preservation surgery was based exclusively on patient age and the radiographic evaluation of hip osteoarthritis.
The study carried out by Griffin et al.(21) aimed to reach multidisciplinary agreement with international experts on the diagnosis and treatment of femoroacetabular impingement. Consensus determined that in order to establish the diagnosis, the patients must present consistent symptoms, positive clinical signs and imaging findings. Adequate treatments in turn comprised conservative management, rehabilitation and arthroscopic or open surgery.
The consensus published by Marín-Peña et al.(24) addressed the indications of hip arthroscopy in degenerative disease of the hip and in hip dysplasia, even contributing surgical "tricks".
The study carried out by Radha et al.(25) addressed hip preservation in femoroacetabular impingement, in particular, intraoperative management of the capsule, the labrum, cartilage defects, the round ligament and bone impingement.
The purpose of the study by Lynch et al.(26) was to develop pre-, intra- and postoperative recommendations for femoroacetabular impingement through evidence-based consensus via a meta-analysis, a systematic review and a group of arthroscopists. Consensus was reached to the effect that hip arthroscopy should be the standard of care for the surgical treatment of classical or arthroscopically accessible femoroacetabular impingement.
The main objective of the study carried out by Winiger et al.(28) was to identify the key variables for performing arthroscopic treatment in femoroacetabular impingement syndrome. Consensus emphasised that treatment of the labrum and correction of the cam-type deformity are the two key elements in hip arthroscopy for the management of femoroacetabular impingement syndrome.
The international consensus of orthopaedic infections addressed the prevention and reduction of risks in the study of Aalirezaie et al.(23) and the treatment and surgical techniques in the study of Abouljoud et al.(22). However, the information on the methodology of these studies appears in the editorial of the monograph dedicated to the international consensus on orthopaedic infections(57). The consensus indicated that there is no evidence that prior arthroscopy increases the risk of subsequent periprosthetic joint infections.
Randelli et al.(20) aimed to establish agreement upon recommendations for the management of thromboembolism in orthopaedic and trauma surgery. They reported that, in all patients requiring pharmacological antithrombotic preventive measures, it is advisable to evaluate both thrombotic risk and bleeding risk — identifying high risk patients and those who will need careful evaluation.
The healthcare technologies evaluation report published by Griffin et al.(30) developed screening criteria for randomised clinical trials. In a survey of clinicians, the latter expressed their reserves about being able to conduct a clinical trial in patients with femoroacetabular impingement, though they were in favour of being able to randomise.
Quality assessment
With regard to quality assessment, of the 13 included studies, those that made use of formal methods obtained better scores, in general terms. In this respect, three of them could be considered of high quality(25,26,29), four of moderate quality(21,27,28,31) and two of low quality(22,23). In contrast, the four studies that used informal or non-structured methods were assessed as being of low quality(19,20,24,30). In all but the studies of Abouljoud et al.(22) and Aalirezaie et al.(23), the purpose or objective of the investigation was clearly defined. With the exception of the studies by Radha et al.(25), Lynch et al.(26), Winiger et al.(28) and Molina et al.(29), none of the publications explained how anonymity was maintained. The details referred to the evaluation of the quality of the studies are found in Table 4.
Discussion
The credibility and usefulness of the results of a consensus are directly proportional to the rigour applied in preparing and conducting the consensus. Consensus methods inevitably must be performed with great methodological rigour and complying with a series of quality requirements. In this respect, we have seen that most of the studies that used a formal or structured consensus method presented greater methodological quality, while in contrast those methods based on informal consensus strategies lacked this high expected quality.
In simpler terms and taking into account that each formal consensus technique can exhibit numerous variants, the main characteristics of the four methods presented can be described as follows. In the Delphi method the participants are surveyed in different rounds. They receive a questionnaire, and individual and/or group feedback is provided on the scores between rounds, specifying their positions and the global positions of the group. Consensus is obtained by means of a mathematical procedure involving the simple summing of individual judgements and the elimination of extreme (outlier) positions. The participants never meet or interact directly(5,10). The number of modifications implemented in the Delphi method has led to considerable confusion about its application(58,59).
In the nominal group technique, the participants physically come together in a meeting directed by an experienced moderator(60). In this meeting, and in an extremely formalised manner, they present their ideas, individually define their points of view, explain their differences, and individually vote each proposed solution(5,10,61). As in the previous case, consensus is obtained by means of a mathematical procedure involving the simple summing of individual judgements.
Consensus conferences involve the evaluation of the available evidence referred to some diagnostic or therapeutic intervention before a jury composed of experts and non-experts that are required to issue a report with recommendations on the use of the intervention. The process simulates an oral hearing in court. During the session, the experts defend the conclusions drawn from the evidence and interact with the public invited to the conference. In 2013, the Office of Disease Prevention withdrew the consensus conferences programme(8), though it is still conducted by other investigators.
Lastly, the RAND/UCLA method begins in a first phase or round with the submission of a questionnaire, while in a second round an in-person meeting is held to clarify or discuss the appropriate or inappropriate use of a medical or surgical procedure(9,61).
The findings of this review show that most of the 13 studies synthesised in the tables of our results were carried out in the United States (6 publications)(22,23,26,27,28,31), with Spain(19,24,29) and the United Kingdom(21,25,30) being the European countries with the greatest scientific production (3 publications), followed by Italy(20) (1 publication). This circumstance could be attributed to the existence of a greater tradition in the use of consensus methods over the years, and since the 1940s, on the part of the United States Army and Air Force(62).
It should be noted that the setting in which the identified consensuses were developed was predominantly at national level in the United States – comprising all the identified publications(26,27,28,31), except two of the same study with an international character(22,23) – while in Europe we located three publications in the United Kingdom(21,25,30) and two in Spain(19,24) involving an international setting. Only one publication in Spain(28) and another in Italy were characterised by a national setting(20). This could be due to the interest in obtaining consensuses applicable to a broader setting and not circumscribed to a single country (in the case of Europe).
Likewise, the identified publications included experts whose number ranged widely from 6 to 869 professionals. Most of them were traumatologists with experience in hip surgery, i.e. from a single discipline. In only four publications(20,21,27,31) did the professionals participating in the consensuses have different types of training (multidisciplinary).
Furthermore, most of the publications(19,21,22,23,24,26,27,29,31) identified the holding of at least one in-person meeting, thereby reflecting the importance of discussion among experts in establishing useful conclusions and consensuses.
The results of this systematic review also show that all the studies that employed informal or non-structured consensus methods(19,20,24,30) in relation to the use of hip arthroscopy, and two of those that used the Delphi technique(22,23), were carried out without clearly defining how consensus was agreed. Therefore, when the authors conclude that the results of the study reflect consensus-based opinion, it seems that the achievement of consensus was assumed as an integral part of the method used. Although consensus may be the expected result of applying a consensus method, we believe that it is necessary to better define the criteria for reaching such consensus and to document the degree of agreement along with the results obtained.
Even though most of the studies included in our systematic review had consensus as an objective, only some of them defined consensus with a specific criterion(21,25,27,28,29,31). Furthermore, this criterion was the reason for termination of the process, normally on the basis of a definition established a priori(21,25,26,27,28,29,31). However, we believe that an adequate approach would be to establish an a priori formal definition of the criteria used for consensus, instead of assuming the latter as an automatic outcome due to the intrinsic fact of making use of a consensus method. Furthermore, the investigators should also specify alternative criteria for termination of the process, including possibly a maximum number of contemplated rounds. If the studies are to be made in the course of a certain number of rounds, the authors should specify how the degree of agreement is going to be quantified at the end of the study.
To the best of our knowledge, there are no validated quality indicators for studies involving consensus methods. We therefore resorted to the recommendations of Humphrey-Murto et al.(18). These indicators were selected on the basis of those which we believe would allow the study to have both internal and external validity. According to these indicators, the quality of the reviewed studies was generally moderate or high in the publications involving formal consensuses. However, it is important to recognise that this scoring is based more on what is reported in the study than on the quality of the study as such. Therefore, we propose that these or other similar criteria should constitute a set of suggested elements to be included in all publications involving consensus methodology. We consider that the applicability of these criteria in the publications would be useful for the diffusion of quality protocols for clinical practice.
These considerations acquire importance due to the fact that level V evidence (expert opinion) remains a necessary component in the methodological repertoire used to determine the response to a clinical question, particularly in situations characterised by the absence of high quality evidence (and by the difficulty of obtaining such evidence), and by clinical variability.
Medicine based on evidence classifies randomised clinical trials and meta-analyses as the highest-ranking evidence, while less relevance is attributed to expert opinion, which is classified as corresponding to the lowest category. Nevertheless, randomised clinical trials and meta-analyses have weaknesses and strengths (since no research method is perfect), and they cannot always be applied or used as a design to obtain investigational results, due to type of patients involved (such as frail individuals or children), or the type of intervention under study (e.g. surgeries) — since doing so would not be acceptable from the ethical perspective.
Indeed, "no study design is perfect, and contradictory findings may arise from all types of studies"(63). Having said this, the practical alternatives to studies where strong confidence has been placed on their results (randomised clinical trials, cohorts, observational studies, etc. with good designs) range from the current observational studies (since we are now in the era of big data in large health registries) to the traditional methods — including expert opinions (more feasible and accessible in some cases).
Whichever design is considered more appropriate for achieving the objective of our research, it must be accompanied by quality and methodological rigour in order to be able to rely upon and extrapolate the results with the lowest risk of biases or limitations. All scientific research is fundamentally dependent upon the use of adequate and rigorously detailed investigational methods — and studies based on expert opinions are no exception to this(64). It may be pertinent to present studies that use consensus methods in accordance with certain indicators similar to those of the CONSORT statement, as used for example in randomised controlled trials.
This systematic review of the literature offers an overview of the different consensus methods used in hip arthroscopy. However, the limitations of the present study are those inherent to the application of its methodology, including publication bias derived from the fact that many scientific studies are not ultimately published, or selection bias, which depends on the objectiveness of the inclusion and exclusion criteria used in the studies. We have minimised the risk of such biases by including several literature sources and working with broad criteria for the inclusion of studies.
Conclusions
The consensus methods analysed in this review and which evaluated the use of arthroscopy in hip disease were predominantly formal consensus protocols. In most cases, the use of these structured methods provided the criteria needed to establish consensus among the professionals.
Tablas
Figuras
Información del artículo
Cita bibliográfica
Autores
Juan Máximo Molina Linde
Área de Evaluación de Tecnologías Sanitarias-AETSA. Fundación Pública Andaluza Progreso y Salud-FPS. Sevilla
Ana María Carlos Gil
Secretaría General de Investigación, Desarrollo e Innovación. Consejería de Salud y Familias. Sevilla
Agencia de Evaluación de Tecnologías Sanitarias de Andalucía (AETSA). Sevilla
Rebeca Isabel Gómez
Área de Evaluación de Tecnologías Sanitarias-AETSA. Fundación Pública Andaluza Progreso y Salud-FPS. Sevilla
Juan Ramón Lacalle Remigio
Departamento de Medicina Preventiva. Universidad de Sevilla
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved human or animal experimentation.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed, and that all the patients included in the study have received sufficient information and have given written informed consent to participation in the study.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Skrabanek P. Nonsensus consensus. Lancet. 1990;335(8703):1446-7.
-
2Thorndike EL. A constant error in psychological ratings. J Appl Psychol. 1920;4(1):25-9.
-
3Nadeau R, Cloutier E, Guay JH. New evidence about the existence of a bandwagon effect in the opinion formation process. Int Political Sci Rev. 1993;14(2):203-13.
-
4Briones E, Marín I, Álvarez R, et al. Fundamentos de consenso en el ámbito de las Ciencias de la Salud. En: Barrera de Unamuno A, Marín León I, Álvarez Gil R. (eds). Metodología de expertos. Consenso en Medicina. Granada: Monografías EASP; 1996. pp. 13-24.
-
5Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):1-88.
-
6Dalkey NC, Helmer O. An experimental application of the Delphi method to the use of experts. Management Science. 1963;9(3):458-67.
-
7Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466-92.
-
8NIH Consensus Development Program [Internet]. U.S. Department of Health & Human Services. National Institutes of Health. Retirement of the National Institutes of Health Consensus Development Program; [citado julio 2022]. Disponible en: https://consensus.nih.gov/default.htm.
-
9Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica: RAND Corporation; 2001.
-
10Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376-80.
-
11Vernon W. The Delphi technique: a review. Int J Ther Rehabil. 2009;16(2):69-76.
-
12Neugebauer EA, Waydhas C, Lendemans S, Rixen D, Eikermann M, Pohlemann T. The treatment of patients with severe and multiple traumatic injuries. Dtsch Arztebl Int. 2012;109(6):102-8.
-
13Genevay S, Courvoisier DS, Konstantinou K, et al. Clinical classification criteria for neurogenic claudication caused by lumbar spinal stenosis. The N-CLASS criteria. Spine J. 2018;18(6):941-7.
-
14Berven SH, Kamper SJ, Germscheid NM, et al. An international consensus on the appropriate evaluation and treatment for adults with spinal deformity. Eur Spine J. 2018;27(3):585-96.
-
15Grypdonck L, Aertgeerts B, Luyten F, et al. Development of quality indicators for an integrated approach of knee osteoarthritis. J Rheumatol. 2014;41(6):1155-62.
-
16Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
-
17Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
-
18Humphrey-Murto S, Varpio L, Gonsalves C, et al. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach. 2017;39(1):14-9.
-
19Altman RD, Bloch DA, Dougados M, et al. Measurement of structural progression in osteoarthritis of the hip: the Barcelona consensus group. Osteoarthritis Cartilage. 2004;12(7):515-24.
-
20Randelli F, Romanini E, Biggi F, et al. II Italian intersociety consensus statement on antithrombotic prophylaxis in orthopaedics and traumatology: arthroscopy, traumatology, leg immobilization, minor orthopaedic procedures and spine surgery. J Orthop Traumatol. 2013;14(1):1-13.
-
21Griffin DR, Dickenson EJ, O'Donnell J, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50(19):1169-76.
-
22Abouljoud MM, Backstein D, Battenberg A, et al. Hip and Knee Section, Treatment, Surgical Technique: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S445-51.
-
23Aalirezaie A, Arumugam SS, Austin M, et al. Hip and Knee Section, Prevention, Risk Mitigation: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S271-8.
-
24Marín-Peña Ó, Más-Martínez J, Ribera-Zabalbeascoa J, et al. Consenso AEA-LATAM sobre artroscopia de cadera en displasia y patología degenerativa. Rev Esp Artrosc Cir Articul. 2019;26(1):53-63.
-
25Radha S, Hutt J, Lall A, et al. Best practice guidelines for arthroscopic intervention in femoroacetabular impingement syndrome: Results from an International Delphi Consensus Project - Phase 1. J Hip Preserv Surg. 2019;6(4):326-38.
-
26Lynch TS, Minkara A, Aoki S, et al. Best Practice Guidelines for Hip Arthroscopy in Femoroacetabular Impingement: Results of a Delphi Process. J Am Acad Orthop Surg. 2020;28(2):81-9.
-
27Riddle DL, Perera RA. American Academy of Orthopedic Surgeons Appropriate Use Criteria for Hip Preservation Surgery: Variables That Drive Appropriateness for Surgery. Arthritis Care Res (Hoboken). 2020;72(3):405-11.
-
28Wininger AE, Dabash S, Ellis TJ, Nho SJ, Harris JD. The Key Parts of Hip Arthroscopy for Femoroacetabular Impingement Syndrome: Implications for the Learning Curve. Orthop J Sports Med. 2021;9(6):23259671211018703.
-
29Molina-Linde JM, Carlos-Gil AM, García-Benítez B, et al.; grupo de panelistas. Artroscopia de cadera. Indicaciones de uso adecuado. Sevilla: Agencia de Evaluación de Tecnologías Sanitarias de Andalucía. Red Española de Agencias de Evaluación de Tecnologías Sanitarias y Prestaciones del SNS; 2017.
-
30Griffin D, Wall P, Realpe A, et al. UK FASHIoN: feasibility study of a randomised controlled trial of arthroscopic surgery for hip impingement compared with best conservative care. Health Technol Assess. 2016;20(32):1-172.
-
31American Academy of Orthopaedic Surgeons. Management of Osteoarthritis of the Hip. Appropriate Use Criteria [Internet]. Illinois: AAOS; 2017 [citado jul 2022]. Disponible en: https://www.aaos.org/ globalassets/qualityand-practice-resources/osteoarthritis-of-the-hip/oa-hip-auc.pdf.
-
32Bick RL, Haas S. Thromboprophylaxis and thrombosis in medical, surgical, trauma, and obstetric/gynecologic patients. Hematol Oncol Clin North Am. 2003;17(1):217-58.
-
33Bounameaux H. Thromboprophylaxis in 'minor' orthopedic surgery. Pathophysiol Haemost Thromb. 2010;37(Suppl. 1):26.
-
34Garcia FL, Williams BT, Maheshwer B, et al. Pain management practice patterns after hip arthroscopy: an international survey. J Hip Preserv Surg. 2020;7(3):537-46.
-
35Griffin D, Wall PDH. Non-operative treatment of femoroacetabular impingement: Design of a fair comparator for a multi-center national randomized trial of arthroscopic surgery. 2012;28(6):e55-6.
-
36Kemp JL, Beasley I. 2016 international consensus on femoroacetabular impingement syndrome: the Warwick Agreement-why does it matter? Br J Sports Med. 2016;50(19):1162-3.
-
37Lall AC, Annin S, Chen JW, et al. Consensus-based classification system for intra-operative management of labral tears during hip arthroscopy-aggregate recommendations from high-volume hip preservation surgeons. J Hip Preserv Surg. 2020;7(4):644-54.
-
38Maldonado DR, Lall AC, Walker-Santiago R, et al. Hip labral reconstruction: consensus study on indications, graft type and technique among high-volume surgeons. J Hip Preserv Surg. 2019;6(1):41-9.
-
39Pohlemann T, Culemann U. Summary of controversial debates during the 5th "Homburg Pelvic Course" 13-15 September 2006. Injury. 2007;38(4):424-30.
-
40Prisco D. Italian Society on Haemostasis and Thrombosis and the Italian Orthopedic Societies: an alliance to improve the standard of venous thromboembolism prophylaxis. Thromb Res. 2014;134(Suppl. 2):S1.
-
41Lynch TS, Minkara A, Aoki SK, et al. Best Practice Guidelines for Hip Arthroscopy in Femoroacetabular Impingement: Results of a Delphi Process. J Am Acad Orthop Surg. 2019,7(7)(suppl 5):2325967119S00318.
-
42Rillo O, Riera H, Espinosa-Morales R, et al. Panlar Consensus on Hand, Hip and Knee OA. Arthritis Rheumatol. 2015;67: (suppl 10).
-
43Aalirezaie A, Anoushiravani A, Cashman J, et al. General Assembly, Prevention, Host Risk Mitigation - Local Factors: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2):S37-S41.
-
44Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (Guidelines according to scientific evidence). Int Angiol. 2006;25(2):101-61.
-
45Conrozier T, Monfort J, Chevalier X, et al. EUROVISCO Recommendations for Optimizing the Clinical Results of Viscosupplementation in Osteoarthritis. Cartilage. 2020;11(1):47-59.
-
46McClincy MP, Wylie JD, Williams DN, et al. Standardizing the Diagnostic Evaluation of Nonarthritic Hip Pain Through the Delphi Method. Orthop J Sports Med. 2021;9(4): 2325967121991213.
-
47Misiolek H, Zajaczkowska R, Daszkiewicz A, et al. Postoperative pain management - 2018 consensus statement of the Section of Regional Anaesthesia and Pain Therapy of the Polish Society of Anaesthesiology and Intensive Therapy, the Polish Society of Regional Anaesthesia and Pain Therapy, the Polish Association for the Study of Pain and the National Consultant in Anaesthesiology and Intensive Therapy. Anest Intens Ther. 2018;50(3):173-99.
-
48Mosler AB, Kemp J, King M, et al. Standardised measurement of physical capacity in young and middle-aged active adults with hip-related pain: recommendations from the first International Hip-related Pain Research Network (IHiPRN) meeting, Zurich, 2018. Br J Sports Med. 2020;54(12):702-10.
-
49Reiman MP, Thorborg K, Covington K, et al. Important clinical descriptors to include in the examination and assessment of patients with femoroacetabular impingement syndrome: an international and multi-disciplinary Delphi survey. Knee Surg Sports Traumatol Arthrosc. 2017;25(6):1975-86.
-
50Reiman MP, Agricola R, Kemp JL, et al. Consensus recommendations on the classification, definition and diagnostic criteria of hip-related pain in young and middle-aged active adults from the International Hip-related Pain Research Network, Zurich 2018. Br J Sports Med. 2020;54(11):631-41.
-
51Takla A, O'Donnell J, Voight M, et al. The 2019 International Society of Hip Preservation (ISHA) physiotherapy agreement on assessment and treatment of femoroacetabular impingement syndrome (FAIS): an international consensus statement. J Hip Preserv Surg. 2020;7(4):631-42.
-
52Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-62.
-
53Molina Linde JM, Carlos Gil AM, Benot López S, et al. Criterios de uso adecuado de la artroscopia de cadera en artrosis. Rev Esp Artrosc Cir Articul. 2018;25(3):236-45.
-
54Molina-Linde JM, Carlos-Gil AM, Benot-López S, et al. Desarrollo de criterios de uso adecuado para la artroscopia de cadera en pacientes con choque femoroacetabular. Rev Esp Cir Ortop Traumatol. 2018;62(5):328-36.
-
55Della Rocca G, Danelli G, Randelli F, et al. II Italian intersociety consensus statement on antithrombotic prophylaxis in orthopedics and traumatology. Minerva Anestesiol. 2013;79(7):778-92.
-
56Hospach T, Hedrich C, Fernández F, et al. Bacterial arthritis in children and adolescents, core theme treatment. Results of the Worlitz consensus meeting 2016, part 2. Monatsschr Kinderheilkd. 2018;166(3):239-48.
-
57Parvizi J, Gehrke T, Mont MA, et al. Introduction: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S1-S2.
-
58Crisp J, Pelletier D, Duffield C, et al. The Delphi method? Nurs Res. 1997;46(2):116-8.
-
59Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008-15.
-
60Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum. 2011;41(2):95-105.
-
61Campbell SM, Cantrill JA. Consensus methods in prescribing research. J Clin Pharm Ther. 2001;26(1):5-14.
-
62Dayé C. How to train your oracle: The Delphi method and its turbulent youth in operations research and the policy sciences. Soc Stud Sci. 2018;48(6):846-68.
-
63Frieden TR. Evidence for Health Decision Making - Beyond Randomized, Controlled Trials. N Engl J Med. 2017;377(5):465-75.
-
64Tonelli MR. In defense of expert opinion. Acad Med. 1999;74(11):1187-92.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- <em>REACA</em>, the journal of everyone
- Applicability of consensus methods in hip arthroscopy: a systematic review
- Evolution of the indication of arthroscopic treatment for femoroacetabular impingement and of the indication of total hip arthroplasty in patients under 60 years of age
- Evolution of glenohumeral bone defects following failed arthroscopic Bankart repair. Study of a case series
- Important articles for the surgical treatment of lateral ankle instability
- Posterolateral elbow instability with osteochondral defect of the <em>capitellum</em>; how to deal with this combination?
- Arthroscopic treatment of a femoral osteochondroma as a cause of femoroacetabular impingement. A case report
- Arthrofibrosis in the mirror
Más en PUBMED
Más en Google Scholar


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.