Pathophysiology, diagnosis and treatment of Achilles tendinopathy
Fisiopatología, diagnóstico y tratamiento de la tendinopatía aquílea
Resumen:
El tendón de Aquiles es el tendón más grande, grueso y fuerte del cuerpo. Por ello, queremos resaltar la importancia de su patología inflamatoria, degenerativa y traumática, que representa un reto diagnóstico y terapéutico. A este respecto, cabe destacar la evolución de la ecografía como método diagnóstico comparable a la resonancia magnética (RM) en muchos aspectos. A tal efecto, la ecografía se ha integrado en los procesos terapéuticos invasivos no quirúrgicos, así como en cirugías endoscópicas, ya que les presta un soporte útil aumentando el campo de visión. En cuanto al tratamiento, sigue siendo de elección el conservador, que asocia medidas físicas y ortopédicas. El fracaso del mismo puede complementarse con terapias invasivas no quirúrgicas como la electrolisis percutánea intratisular, así como la infiltración de diferentes sustancias. El tratamiento quirúrgico solo está indicado ante la ausencia de resultados beneficiosos de los anteriores y con el único objetivo de restablecer la función del tendón. En esta línea, se han desarrollado técnicas poco invasivas con resultados similares o incluso mejores que los obtenidos con cirugía abierta, con una menor morbilidad y tiempo quirúrgico.
Abstract:
The Achilles tendon is the largest, thickest and strongest tendon in the body. It is thus advisable to underscore the importance of the inflammatory, degenerative and traumatic disorders of this tendon, which constitute a diagnostic and therapeutic challenge. In this regard, mention must be made of the evolution of ultrasound as a diagnostic method comparable in many aspects to magnetic resonance imaging (MRI). Ultrasound has become incorporated to nonsurgical invasive therapeutic processes, as well as to endoscopic surgery, since it offers useful support, magnifying the field of vision. With regard to treatment, conservative management remains the preferred option, combining physical and orthopedic measures. In the event of failure of such treatment, nonsurgical invasive procedures may be used, such as intratissue percutaneous electrolysis, as well as the infiltration of different substances. Surgery is only indicated when the above measures fail to yield benefits, and with the sole purpose of restoring tendon function. In this regard, scantly invasive techniques have been developed offering outcomes similar to or even better than those obtained by open surgery, and with less morbidity and shorter operating times.
Introduction
The Achilles tendon is the most powerful tendon in the body, being able to resist traction forces up to 10 times body weight(1). Given the importance of this tendon, it is of interest to underscore its pathophysiological mechanisms, as they can help us understand its great clinical diversity from the inflammatory, degenerative and traumatic perspectives(2,3,4,5). From the diagnostic point of view, the development of ultrasound has provided an important tool, comparable in many aspects to magnetic resonance imaging (MRI)(2). In addition, ultrasound plays a significant role by affording imaging assistance to nonsurgical invasive processes and endoscopic surgeries, expanding the field of vision of these surgical techniques(6,7). Conservative management should be the first-line choice in patients with tendinopathy, and in this regard physical therapies are currently of particular relevance(8,9). These measures may be combined with invasive procedures in the nonsurgical setting, such as intratissue percutaneous electrolysis (IPE)(10,11) or the infiltration of different substances presently available(12,13,14,15). With regard to surgical treatment, the objective is to restore the previous function of the tendon. Of note in recent years has been the development of minimally invasive and endoscopic techniques, as they are effective and result in lesser morbidity, shorter operating times and fewer complications(16,17,18,19,20,21,22,23).
The present review was carried out to describe the natural course of tendinopathies, underscoring the diagnostic role of ultrasound and MRI, and to examine the imaging findings that define each disease condition. Lastly, an analysis is made of the principles of treatment, covering the range from the most conservative options to the most invasive strategies.
Pathophysiology and natural course of Achilles tendinopathy
The Achilles tendon is the largest, thickest and strongest tendon in the body. It measures 15 cm in length, 12-15 mm in width and 5-6 mm in thickness. The tendon is exposed to tensile forces equivalent to up to 10 times body weight(1).
Disease of the Achilles tendon can be divided into two large groups(2,5): tendinopathies and tendon ruptures.
• Tendinopathies. In this group we can distinguish the following:
– Peritendinitis: paratendon inflammation, characterized by the proliferation of fibroblasts and hypervascularisation. It can be acute (< 2 weeks), subacute (2-6 weeks) or chronic (> 6 weeks). Chronic cases may be associated with degenerative phenomena.
– Tendinosis: degeneration of the tendon. This condition is secondary to repeated microtraumatisms(3), aging, or a combination of both. Tenocyte metabolism is altered in such cases, with the production of less resistant type 3 collagen and an altered matrix component. Catabolism is increased (early apoptosis) and an excessive repair response is observed, with neoangiogenesis, neoinnervation (pain) and poor quality healing, giving rise to degenerative changes. These disorders in turn can evolve towards interstitial ruptures, partial ruptures or acute complete ruptures.
Based on their location, tendinopathies can in turn be classified as follows:
– Insertional tendinopathy(24): tendon disease at its insertion site. This is often associated to retroachilles bursitis. In this context, Haglund deformity is not necessarily associated to this condition, and is certainly not its cause(25).
– Non-insertional tendinopathy(26): the disease mechanisms are found in the body of the tendon, which is a poorly vascularized zone and thus susceptible to degenerative changes. This condition is usually associated to microruptures when overuse mechanisms are present. The presence of this type of disease is less common at the myotendinous junction, which is more related to muscle tearing phenomena.
A number of risk factors intervene in the development of Achilles tendinopathy. These include:
– Intrinsic factors: anatomical features predisposing to biomechanical disability to absorb forces, and physiological aspects that affect the tissue resistance of the tendon(27). Mention can be made of the following: excessive varus/valgus of the rearfoot or forefoot, subtalar hyperpronation, cavus foot, dysmetria, musculotendinous atrophy or deficiency, muscle imbalance, overweight and obesity, advanced age and rheumatic diseases involving inflammatory arthropathy or tendinosis.
– Extrinsic factors(28): these include overuse, excessive sports activity, training errors (duration, intensity), running or jumping on inadequate surfaces(29), inappropriate footwear, and drugs such as fluoroquinolones and corticosteroids (systemic or as local infiltrations).
• Tendon ruptures: these injuries are more common in males (5:1), in individuals between 30-50 years of age, and in non-professional athletes. The incidence increases in relation to sports activity at older ages. These injuries are usually a consequence of indirect trauma, with the combination of mechanical tension and intratendinous degeneration, secondary to sudden dorsiflexion of the ankle starting from active plantar flexion with the knee extended or in forced plantar flexion starting from a neutral position(4).
Rupture may be partial, complete and intrasubstance. Complete rupture is the most common presentation. These lesions are generally associated to degenerative phenomena of the tendon; the risk factors are therefore usually similar to those described above in relation to tendinopathies(28).
Imaging diagnosis
Ultrasound
Ultrasound allows us to study the tendon over its entire length, visualizing a fibrillar pattern along the longitudinal axis and an oval hyperechogenic structure on the transverse axis(6,30).
A high-frequency (10-18 MHz) linear probe is used. It is advisable to start the exploration at the transverse axis proximal to the myotendinous junction and in a distal direction. Posteriorly, we assess the tendon along the longitudinal axis, rotating the probe 90°(30).
Ultrasound exploration of the normal Achilles tendon shows how distal insertion in the calcaneus takes place via a 2-cm hypoechogenic fibrocartilaginous interface. Likewise, the paratendon (not always visible) most often appears as a hyperechogenic band in the posterior region and as a more hypoechogenic structure in the lateral and medial zone(30). Kager's fat pad is identified as a hypoechogenic inverted triangle ahead of the Achilles tendon(6). In the study of the bursae, the retrocalcaneal bursa physiologically may contain fluid, though measuring no more than 3 mm, and the retroachilleal bursa is normally not visible(5).
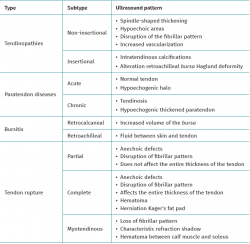
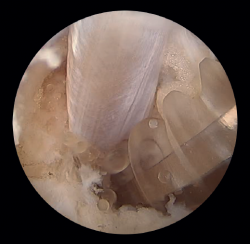
With regard to the pathological ultrasound findings (Table 1), non-insertional Achilles tendinopathy (2-6 cm from the calcaneal insertion) (Figure 1) is characterized by a spindle-form thickening of the tendon area, hypoechoic areas and disruption of the fibrillar pattern that can correspond to degeneration or partial rupture(24,28). Increased vascularization of the peritendinous ventral portion may also be present(5,6,30).
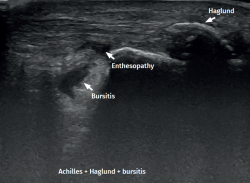
However, in the chronic forms of insertional Achilles tendinopathy or enthesopathy (present in the distal 2 cm of the tendon), we can observe calcifications within the tendon in the form of traction enthesophytes or at the insertion site in the form of spurs. These conditions are often associated to alterations of the retroachilleal bursa(5,31) (Figure 2).
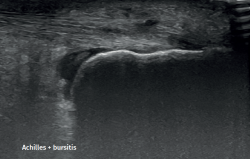
In isolated peritendinitis, ultrasound shows a normal tendon surrounded by fluid in the form of a hypoechogenic halo. In chronic presentations we observe a hypoechogenic thickened paratendon usually associated to tendinosis(32).
Retrocalcaneal bursitis is characterized by an increased volume of the bursa, with the possible association of Haglund deformity(25). In the case of retroachilles or superficial calcaneal bursitis we can identify fluid between the skin and the Achilles tendon(5).
In patients with Achilles tendon ruptures, ultrasound evidences partial ruptures as anechoic defects with discontinuity of the fibrillar pattern along the longitudinal axis of the tendon that does not affect the full thickness of the latter(33). In the case of complete rupture, these anechoic defects are present throughout the whole thickness of the tendon. In situations of acute rupture, we can evaluate the hematoma and herniation of Kager's fat pad. However, in chronic cases, the diagnosis is fundamented upon the MRI findings, as will be detailed below. Myotendinous junction rupture is characterized by a loss of the fibrillar pattern with a typical refraction shadow that delimits the ruptured and retracted segments, together with the presence of an interfascial hematoma located between the calf muscle and the soleus(33).
Magnetic resonance imaging
Despite the evolution of ultrasound in this field, MRI remains useful for diagnosing disorders of the Achilles tendon, since it is able to identify and quantify degeneration, fat infiltration and intratendinous lesions, and is particularly useful for identifying chronic partial and complete ruptures.
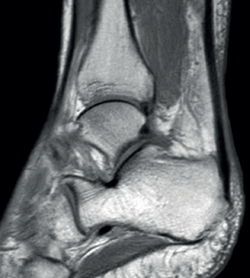
The normal Achilles tendon is observed as a hypointense structure in both T1- and T2-weighted sequences(33) (Figure 3). In the sagittal plane its margins are parallel, and in the axial plane its distal portion presents an anterior concave margin towards the calcaneus over most of its course. The thickness is similar along its trajectory, and a minimum amount of fluid is observed at retrocalcaneal level (fluid surrounding the tendon is pathological)(34).
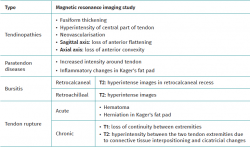
With regard to the pathological patterns of the Achilles tendon (Table 2), MRI visualizes tendinosis as a loss of the normal form of the tendon, with fusiform thickening. Loss of the anterior flattening can be observed in sagittal sequences, together with convexity of the anterior surface of the tendon in axial sequences. We can also observe hyperintense zones in the central part of the tendon in both T1- and T2-weighted sequences, with tendon neovascularization(34). It must be taken into account that signs of Achilles tendinosis can be found in asymptomatic individuals(35).
Paratendon disease is characterized by increased intensity in both T1- and T2-weighted sequences around the tendon, with the possible association of inflammatory changes in Kager's fat pad(34).
Retrocalcaneal bursitis is in turn characterized by hyperintense images in T2 sequencing in the retrocalcaneal recess(5), while in retroachilles bursitis or superficial calcaneal bursitis, inflammation of the bursa results in hyperintense images in T2 sequencing(5).
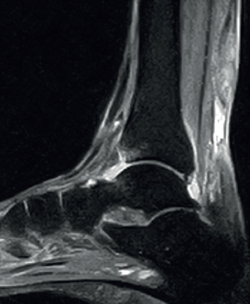
Lastly, in acute Achilles tendon rupture, MRI shows the hematoma and also herniation of Kager's fat pad - these signs being similar to those identified by ultrasound (Figure 4). In chronic ruptures, MRI is the best diagnostic tool. Loss of continuity between the extremities is observed in T1 sequencing, while T2 imaging evidences hyperintensity between the two tendinous extremities due to the interpositioning of connective tissue and cicatricial changes(36).
Nonsurgical treatment
The different possible clinical presentations of Achilles tendinopathy make it necessary to adjust treatment to each individual case, identifying the problem and adopting the opportune measures to secure healing and an optimum return to physical activity(37).
Conservative treatment should be regarded as the first-line treatment strategy. The first step should be the identification and correction of mobility disorders, stepping problems and misalignments or dysmetria(27).
Prolonged splint immobilization has been shown to exert negative effects upon the tendon tissue(37). Intermittent splint immobilization associated to a physical therapy program likewise has not evidenced superiority with respect to physical therapy alone(38,39).
Foot orthoses may afford benefit because they reduce mechanical stress by correcting misalignments(40), increase rearfoot mobility(41), and avoid weight bearing of the Achilles tendon(42). However, a meta-analysis of randomized clinical trials found no benefits with the use of orthoses associated to physical therapy programs or isolatedly compared against placebo. Their use in disease conditions of this kind is therefore not advised(43).
With regard to the physical therapy options, those based on exercise have been of special relevance for over three decades. Eccentric exercises have been regarded as the basis for the treatment of most tendinopathies of the lower extremities, particularly since the publication of the protocol developed by Alfredson(44), involving a training program based on high frequency and low intensity exercises on an inclined surface. Its effectiveness in improving pain and function, as well as tendon structure, has been widely established for both insertional and non-insertional tendinopathies(8). Although some studies have described better outcomes than those obtained with concentric exercises considered isolatedly(45), the difference between the different types of contraction in the treatment of Achilles tendinopathy is currently not so clear(46). However, the introduction of work programs combining different exercises performed at variable intensities, such as heavy, slow resistance training, has evidenced outcomes comparable to those of eccentric training only. As a result, the combination of concentric and eccentric exercises is now often recommended(9).
The use of shockwave therapy for tendinopathy has spread in recent years. A number of mechanisms can explain its beneficial effects in tendon disease, such as the inhibition of afferent nociceptive receptors, and increased local cell proliferation(47). The use of shockwave therapy in insertional Achilles tendinopathy appears to be more effective than eccentric exercises alone over the middle term, while in non-insertional tendinopathy the results are comparable, and in any case better than no treatment(48) Such therapy therefore constitutes a valid alternative in application to these disorders.
Nonsurgical invasive treatments
As has been mentioned, the literature describes many therapeutic algorithms for the treatment of Achilles tendinopathy, ranging from rehabilitation to surgery. The use of invasive puncture techniques constitutes an intermediate strategy. Employing them in combination with imaging methods such as ultrasound increases effectiveness compared with blind puncture and reduces the risk of complications associated to accidental outside-of-target puncture or the effect of the employed material in undesired structures.
The infiltration of corticosteroids has been found to reduce pain, and inflammation over the short term, and improves the ultrasound images of the tendon, probably as a result of its vasoconstrictive effect(49). Important adverse effects have been reported, however, including tendon weakness and rupture(12). Johansen et al.(50) conducted a systematic review with a follow-up period of 10 years in patients treated with ultrasound-guided corticosteroid injections supplemented by controlled rehabilitation. The authors found that one or two corticosteroid injections are effective and safe in treating Achilles tendon pain, without signs of deterioration of the tendon over the long term, in both insertional and non-insertional tendinopathies. Given the heterogeneity of the outcomes, their use is currently subject to controversy, since any benefit afforded by the corticosteroids appears to be outweighed by the potential complications(51).
The use of platelet-rich plasma (PRP) has become another therapeutic alternative. The current scientific evidence does not clearly support its use in Achilles tendinopathy(52), since most of the existing studies have recorded no statistically significant clinical improvement. Yi-Jun Zhang(13) et al. performed a meta-analysis comparing infiltrations with PRP and versus placebo (physiological saline solution [PSS]), both combined with eccentric exercises. They assessed improvement based on the VISA-A scale, changes in the thickness of the tendon, and the Doppler ultrasound findings. No statistically significant results were obtained for any of the studied parameters.
Prolotherapy(53) involves injection into the damaged zone of an irritant (most commonly hyperosmolar dextrose) that induces a local inflammatory response and stimulates tissue repair. In addition, it reduces the neovascularization observed in non-insertional tendinopathies. A recent systematic review(14) has concluded that prolotherapy affords results superior to or at least comparable to those of treatment with eccentric exercises, and is an option to be considered in the treatment of non-insertional disease conditions.
Polydocanol(54) used as sclerotizing therapeutic agent (causing obliteration of the aberrant vascularization of the affected tendon), injected under Doppler ultrasound control over the abnormal vessels on the ventral surface of the Achilles tendon, has shown satisfactory clinical results as reflected by the function and pain scores. A randomized clinical trial(15) comparing polydocanol versus placebo, with assessment of the results after 6 months of follow-up, has shown the active treatment to be safe, but ineffective over the middle term.
Intratissue percutaneous electrolysis (IPE) involves the application of a galvanic current through a needle connected to a cathode, with the purpose of promoting an inflammatory and repair response of the affected tendon. This treatment moreover releases H2, which is a potent free radical inhibitor and promotes the analgesic effect(10). Ronzio(11) et al. carried out a clinical trial comparing rehabilitation therapy versus the latter associated to IPE, in patients with Achilles tendinopathy. The results proved significant in terms of pain reduction, increased joint range and the reduction of morning stiffness, in favour of the second treatment group.
In this same line, percutaneous hydrodissection to release the adherences formed between the Achilles tendon and Kager's fat pad has been proposed. Lulu He et al.(55) treated three patients using a simple hydrodissection technique on an ambulatory basis, releasing these structures and restoring tendon mobility.
Surgical treatment
The aim of surgical treatment is to restore the previous function of the tendon. Surgery is usually indicated once the possibilities of conservative management have been exhausted. Of note in recent years has been the development of minimally invasive and endoscopic techniques, as they are effective and result in lesser morbidity, shorter operating times and fewer complications(16).
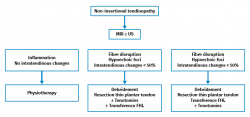
Among the surgical treatment options in non-insertional Achilles tendinopathy, mention must be made (Figure 5)(56) of open debridement of the nodules and adherences, with or without paratendon resection(17). When debridement of the tendon exceeds 50%, augmentation with flexor hallucis longus (FHL) is advised. The literature describes good outcomes in 36-77% of the cases(5,57), though with an incidence of complications of 19-40% in the form of infections, skin necrosis, wound dehiscence, and hypertrophic and/or painful scarring(18).
Surgeries of this kind can also be performed with endoscopic techniques. Described by Van Dijk(58,59), these strategies allow resection of the paratendon and tendon debridement, with good outcomes in 67-97% of the cases. Such techniques allow the elimination of the aberrant innervation and neovascularization with less soft tissue aggression, fewer complications (8.6-19%)(18) and a faster return to sports activities(19,20). In this respect, by performing a smaller incision, we obtain less scar tissue and less postoperative pain(18,21).
The abovementioned techniques can be supplemented by longitudinal tenotomies, following the direction of the fibres of the Achilles tendon to promote neovascularization and repair of the tendon(18).
Transpositioning of the FHL (Figure 6) seeks to improve the pain without loss of strength in plantar flexion of the hallux(22,60). The reported patient satisfaction rate among patients operated upon with this technique is between 73-90%(22).
Van Sterkenburg and Van Dijk(59) described the importance of the thin plantaris tendon in tendinopathy of the middle portion of the Achilles tendon. Under normal conditions, the thin plantaris tendon is a freely moving element. However, adherences form between these two structures in the course of Achilles tendinopathy. In fact, some authors have demonstrated histological changes similar to those found in the Achilles tendon(22,59). Therefore, resection of the thin plantaris tendon is recommended during treatment, since this helps to alleviate the symptoms. In this line, percutaneous thin plantaris release with IPE has been described(10), offering promising results with respect to open surgical release, since the risk of wound complications is lower, and early rehabilitation is facilitated.
In 2011, Duthon et al.(61) combined elongation of the medial gastrocnemius with reduction of loading upon the pathological tendon, allowing healing and symptoms relief. This approach improves the outcomes of endoscopic tenolysis and does not affect the Achilles-calcaneus-plantar system in athletes(62).
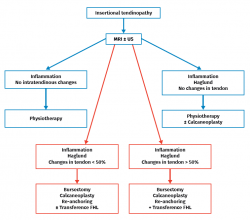
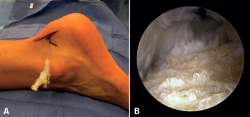
In the course of insertional tendinopathy, 25% of all patients require surgical treatment (Figure 7). Resection of retrocalcaneal bursitis and of the posterior calcaneal tuberosity or calcaneoplasty is a technique that can be performed through open or endoscopic surgery using a lateral viewing portal and a medial operating portal. This procedure is indicated for removing the inflamed bursa caused by friction between the calcaneus and the Achilles tendon even in the absence of tendinopathy(23) (Figures 8A and B). In the presence of tendinopathy, debridement can be carried out, followed by reinsertion of the Achilles tendon, associated or not to transpositioning of the FHL(60). The risk of tendon deinsertion is low when debridement is less than 50%; if over 50%, re-anchoring or augmentation is advised(63). Although excellent outcomes are reported in 84.8% of the patients, with a low incidence of complications (10-15%), a return to initial sports activity does not prove possible in 25% of the cases(17).
Transpositioning of the FHL is usually associated in patients with important Achilles tendon involvement, high physical requirements, a high body mass index, age over 50 years, and with diabetes mellitus (DM), arterial hypertension (AHT) and/or chronic corticosteroid treatment. In this respect, Kenneth et al.(64) carried out a level I study in patients over 50 years of age using an open surgical technique, and recorded significant improvement in plantar flexion strength, without a loss of strength in the hallux. Vega and Vila(16), in a study of chronic Achilles tendon rupture, performed FHL transference assisted by endoscopy and without resection of the tendon scar tissue. Excellent results were obtained, with the advantage afforded by the fact that this was a minimally invasive procedure. However, the technique requires great surgeon experience. The hypothesis of the above authors was that vascularization of the muscle belly of the FHL increases perfusion of the damaged Achilles tendon, and the tendon scar tissue is replaced by healthy tendon or nearly healthy tendon. This phenomenon is also observed in the postoperative MRI scans.
Lastly, mention should be made of the osteotomy of Zadek, involving a closing osteotomy with a dorsal wedge to which resection of the retrocalcaneal bursitis and exeresis of the posterosuperior calcaneal tuberosity can be added. This technique is reserved for selected cases, particularly those with important calcaneal inclination (> 20°)(65) and in patients with insertional calcific tendinopathy(66). Although good outcomes have been reported in 96% of the cases, this treatment widens the heel, requires osteosynthesis material with postoperative avoidance of weight bearing, and is associated to greater variability of the results compared with calcaneoplasty(23). The studies published to date on this procedure are limited, and further investigation is needed.
Emphasis must be placed on the role of ultrasound in surgery of the Achilles tendon. The imaging support afforded by this technique during endoscopic surgery allows greater surgical control, since we have a more complete vision than when only endoscopic surgery is carried out. In this way, iatrogenic damage to the adjacent structures or to the elements where the surgical process itself is carried out tends to be minimized(7). Likewise, and in real time, ultrasound allows a diagnostic assessment of the Achilles tendon that in turn facilitates more precise surgery.
Conclusions
Achilles tendinopathy is a multifactorial condition with different clinical expressions - a fact that complicates the diagnostic and particularly the therapeutic process.
The development of ultrasound has made it a diagnostic tool comparable to MRI. In addition, the capacity to perform dynamic studies with ultrasound has facilitated its use in invasive treatments involving puncture, and in surgery of the Achilles tendon.
Despite the evolution of ultrasound in this field, MRI remains useful for diagnosing disorders of the Achilles tendon, being particularly effective in identifying partial and complete ruptures - particularly those of a chronic nature.
However, it must be underscored that conservative management is the first choice option, with surgery being indicated in those cases where conservative treatment proves ineffective.
Of note in the field of surgery is the development of minimally invasive and endoscopic techniques with the aim of securing more effective procedures, with lesser morbidity and with faster functional recovery.
Tablas
Figuras
Figure 1. Insertional Achilles tendinopathy associated to retroachilleal bursitis and Haglund deformity.
Figure 3. Magnetic resonance imaging view of ankle (T1 sequencing). Sagittal view showing a normal tendon.
Figure 4. Sagittal magnetic resonance imaging view showing subacute non-insertional Achilles tendon rupture. Hyperintensity area between the two extremities.
Figure 6. Transpositioning of the flexor hallucis longus (FHL). Fixation to the calcaneus with biotenodesis screw.
Información del artículo
Cita bibliográfica
Autores
Néstor Antonio Zurita Uroz
Hospital IMED Elche. Alicante
Arthrosport. Zaragoza
Servicios Médicos. Real Federación Española de Natación
Servicio de Cirugía Ortopédica y Traumatología. Hospital IMED Elche. Alicante
Andrea Paniagua González
Unidad de Hombro y Codo. Hospital Fraternidad-Muprespa Habana. Madrid
Unidad de Hombro y Codo. Hospital Universitario Ramón y Cajal. Madrid
Ignacio Fernández Kelly
Hospital Monográfico Asepeyo Coslada. Madrid
Pablo Carnero Martín de Soto
Arthrosport Zaragoza
Instituto Malagueño de Traumatología del Deporte (IMATDE). Málaga
Hospital Regional de Málaga
Dolores Pilar Garrido Pozo
Unidad de Miembro Inferior. Hospital Fraternidad-Muprespa Habana. Madrid
Deborah González-García
Complejo Hospitalario Quirón Juan Bravo. Madrid
Unidad de Pie y Tobillo. Hospital Universitario de Guadalajara
Hospital ASEPEYO. Coslada. Madrid
Ethical responsibilities
Conflicts of interest. The author Néstor A. Zurita Uroz is a compliance to Zimmer Biomet.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved studies in humans or in animals.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1O'Brien M. The anatomy of the Achilles tendon. Foot Ankle Clin. 2005 Jun;10(2):225-38.
-
2Soma CA, Mandelbaum BR. Achilles tendon disorders. Clin Sports Med. 1994;13(4):811-23.
-
3Silbernagel KG, Thomeé R, Thomeé P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain--a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001 Aug;11(4):197-206.
-
4Devereaux MD, Lachmann SM. Athletes attending a sports injury clinic--a review. Br J Sports Med. 1983;17(4):137-42.
-
5Van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N. Terminology for Achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc. 2011 May;19(5):835-41.
-
6Jimenez Díaz. Ecografía musculoesquelética. 1.ª Edición. Marban; 2017. pp. 288-93.
-
7Smith J, Alfredson H, Masci L, Sellon JL, Woods CD. Sonographically Guided Plantaris Tendon Release: A Cadaveric Validation Study. PM R. 2019 Jan;11(1):56-63.
-
8Beyer R, Kongsgaard M, Hougs Kjær B, Øhlenschlæger T, Kjær M, Magnusson SP. Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am J Sports Med. 2015 Jul;43(7):1704-11.
-
9Malliaras P, Barton CJ, Reeves ND, Langberg H. Achilles and patellar tendinopathy loading programmes: a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013 Apr;43(4):267-86.
-
10Mattiussi G, Moreno C. Percutaneous electrochemical debridement of the Plantaris tendon: a novel option in the treatment of midportion Achilles tendinopathy. J Am Podiatr Med Assoc. 2018;108(5):437-41.
-
11Ronzio OA, Da Silva E, Soares MDR, Froes P. Effects of percutaneous microelectrolysis (MEP®) on pain, rom and morning stiffness in patients with Achilles tendinopathy. Eur J Physiother. 2017;19:62-3.
-
12Kearney RS, Parsons N, Metcalfe D, Costa ML. Injection therapies for Achilles tendinopathy. Cochrane Database Syst Rev. 2015 May 26;(5):CD010960.
-
13Zhang YJ, Xu SZ, Gu PC, et al. Is platelet-rich plasma injection effective for chronic Achilles tendinopathy? A meta-analysis. Clin Orthop Relat Res. 2018;476(8):1633-41.
-
14Sanderson LM, Bryant A. Effectiveness and safety of prolotherapy injections for management of lower limb tendinopathy and fasciopathy: a systematic review. J Foot and Ankle Res. 2015;8(1):57.
-
15Ebbesen BH, Molgaard CM, Olesen JL, Gregersen HE, Simonsen O. No beneficial effect of polidocanol treatment in Achilles tendinopathy: a randomized controlled trial. Knee Surg Sport Traumatol Arthrosc. 2018;26(7):2038-44.
-
16Vega J, Vilá J, Batista J, Malagelada F, Dalmau-Pastor M. Endoscopic Flexor Hallucis Longus Transfer for Chronic Noninsertional Achilles Tendon Rupture. Foot Ankle Int. 2018;39(12):1464-72.
-
17Leppilahti J, Karpakka J, Gorra A, Puranen J, Orava S. Surgical treatment of overuse injuries to the Achilles tendon. Clin J Sport Med. 1994;4:100-7.
-
18Chraim M, Alrabai HM, Krenn S, Bock P, Trnka HJ. Short-Term Results of Endoscopic Percutaneous Longitudinal Tenotomy for Noninsertional Achilles Tendinopathy and the Presentation of a Simplified Operative Method. Foot Ankle Spec. 2019 Feb;12(1):73-8.
-
19Maquirriain J. Surgical treatment of chronic Achilles tendinopathy: long-term results of the endoscopic technique. J Foot Ankle Surg. 2013;52:451-5.
-
20Maquirriain J, Ayerza M, Costa-Paz M, Muscolo DL. Endoscopic surgery in chronic Achilles tendinopathies: a preliminary report. Arthroscopy. 2002;18:298-303.
-
21Cerrato R, Switaj P . Using Arthroscopic Techniques for Achilles Pathology. Foot Ankle Clin North Am. 2017;22:781-99.
-
22Opdam KTM, Baltes TPA, Zwiers R, Wiegerinck JI, van Dijk CN. Endoscopic Treatment of Mid-Portion Achilles Tendinopathy: A Retrospective Case Series of Patient Satisfaction and Functional Outcome at a 2- to 8-Year Follow-up. Arthroscopy. 2018 Jan;34(1):264-9.
-
23Scholten PE, van Dijk CN. Endoscopic calcaneoplasty. Foot Ankle Clin. 2006 Jun;11(2):439-46.
-
24Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med. 2004;34(14):1005-17.
-
25Kang S, Thordarson DB, Charlton TP. Insertional Achilles Tendinitis and Haglund’s Deformity. Foot Ankle Int. 2012 Jun;33(6):487-91.
-
26Pearce CJ, Tan A. Non-insertional Achilles tendinopathy. EFFORT Open Rev. 2017;1(11):383-90.
-
27Clement DB, Taunton JE, Smart GW. Achilles tendinitis and peritendinitis: etiology and treatment. Am J Sports Med. 1984;12(3):179-84.
-
28Kujala UM, Sarna S, Kaprio J. Cumulative incidence of Achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-5.
-
29Kvist M. Achilles tendon injuries in athletes. Sports Med. 1994;18(3):173-201.
-
30Iriarte I, Balius R, Cerezal L, Pedret C. Experto universitario en ecografía musculoesquelética. Universidad Francisco de Vitoria. Editorial Panamericana. Curso 2018-2019.
-
31Caudell GM. Insertional Achilles Tendinopathy. Clin Podiatr Med Surg. 2017;34:195-205.
-
32Stecco A, Busoni F, Stecco C, et al. Comparative ultrasonographic evaluation of the Achilles paratenon in symptomatic and asymptomatic subjects: an imaging study. Surg Radiol Anat. 2015 Apr;37(3):281-5.
-
33Cobos-Huerga C, Vega ML, Anguita G, Martín A. Lesiones del tendón de Aquiles. Diagnóstico por imagen. Rev Int Cienc Podol. 2011;5(2):35-45.
-
34Bäcker HC, Wong TT, Vosseller JT. MRI Assessment of Degeneration of the Tendon in Achilles Tendon Ruptures. Foot Ankle Int. 2019 Aug;40(8):895-9.
-
35Maffulli N, Via AG, Oliva F. Chronic Achilles Tendon Rupture. Open Orthop J. 2017 Jul 31;11:660-9.
-
36Paavola M, Kannus P, Paakkala T, Pasanen M, Järvinen M. Long-term prognosis of patients with Achilles tendinopathy. An observational 8-year follow-up study. Am J Sports Med. 2000 Sep-Oct;28(5):634-42.
-
37Loitz BJ, Zernicke RF, Vailas AC, Kody MH, Meals RA. Effects of short-term immobilization versus continuous passive motion on the biomechanical and biochemical properties of the rabbit tendon. Clin Orthop Relat Res. 1989 Jul;(244):265-71.
-
38Knobloch K, Schreibmueller L, Longo UG, Vogt PM. Eccentric exercises for the management of tendinopathy of the main body of the Achilles tendon with or without the AirHeel Brace. A randomized controlled trial. A: effects on pain and microcirculation. Disabil Rehabil. 2008;30(20-22):1685-91.
-
39Petersen W, Welp R, Rosenbaum D. Chronic Achilles tendinopathy: a prospective randomized study comparing the therapeutic effect of eccentric training, the AirHeel brace, and a combination of both. Am J Sports Med. 2007 Oct;35(10):1659-67.
-
40Maffulli N, Kader D. Tendinopathy of tendo achillis. J Bone Joint Surg Br. 2002 Jan;84(1):1-8.
-
41Donoghue OA, Harrison AJ, Coffey N, Hayes K. Functional data analysis of running kinematics in chronic Achilles tendon injury. Med Sci Sports Exerc. 2008 Jul;40(7):1323-35.
-
42Hertel J, Sloss BR, Earl JE. Effect of foot orthotics on quadriceps and gluteus medius electromyographic activity during selected exercises. Arch Phys Med Rehabil. 2005 Jan;86(1):26-30.
-
43Wilson F, Walshe M, O'Dwyer T, Bennett K, Mockler D, Bleakley C. Exercise, orthoses and splinting for treating Achilles tendinopathy: a systematic review with meta-analysis. Br J Sports Med. 2018 Dec;52(24):1564-74.
-
44Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998 May-Jun;26(3):360-6.
-
45Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):42-7.
-
46Head H, Mallows A, Debenham J, Travers MJ, Allen L. The efficacy of loading programmes for improving patient-reported outcomes in chronic midportion Achilles tendinopathy: a systematic review. Musculoskeletal Care. 2019 Dec;17(4):283-99.
-
47Vitali M, Naim Rodriguez N, Pironti P, et al. ESWT and nutraceutical supplementation (Tendisulfur Forte) vs ESWT-only in the treatment of lateral epicondylitis, Achilles tendinopathy, and rotator cuff tendinopathy: a comparative study. J Drug Assess. 2019 May 3;8(1):77-86.
-
48Korakakis V, Whiteley R, Tzavara A, Malliaropoulos N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med. 2018 Mar;52(6):387-407.
-
49Suzuki T, Nakamura Y, Moriya T, Sasano H. Effects of steroid hormones on vascular functions. Microsc Res Tech. 2003;60:76-84.
-
50Johannsen F, Jensen S, Wetke E. 10-year follow-up after standardized treatment for Achilles tendinopathy. BMJ Open Sport Exerc Med. 2018;4(1).
-
51Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomized controlled trials. Lancet. 2010;376(9754):1751-67.
-
52Poggio D, Álvarez C, García R. Evidencia de los nuevos tratamientos de la cirugía del pie y el tobillo. Monografía SEMCPT. 2017;9:3-12.
-
53Chan O, Havard B, Morton S, et al. Outcomes of prolotherapy por intratendinous Achilles tears: a case series. Muscles Ligaments Tendons J. 2017;7(1):78-87.
-
54Roche AJ, Calder JDF. Achilles tendinopathy: a review of the current concepts of treatment. Bone Joint J. 2013;95B:1299-307.
-
55He L, Genin J, Delzell P. Ultrasound diagnosis and percutaneous treatment of Achilles tendon tethering: a case series. Skeletal Radiol. 2016;45(9):1293-8.
-
56Reddy SS, Pedowitz DI, Parekh SG, Omar IM, Wapner KL. Surgical treatment for chronic disease and disorders of the Achilles tendon. J Am Acad Orthop Surg. 2009 Jan;17(1):3-14.
-
57Maffulli N, Binfield PM, Moore D, King JB. Surgical decompression of chronic central core lesions of the Achilles tendon. Am J Sports Med. 1999;27:747-52.
-
58Zwiers R, Wiegerinck JI, Van Dijk CN. Treatment of midportion Achilles tendinopathy: an evidence‑based overview. Knee Surg Sports Traumatol Arthrosc. 2016 Jul;24(7):2103-11.
-
59Van Sterkenburg MN, Kerkhoffs GM, van Dijk CN. Good outcome after stripping the plantaris tendon in patients with chronic mid-portion Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1362-6.
-
60Staggers JR, Smith K, de C Netto C, Naranje S, Prasad K, Shah A. Reconstruction for chronic Achilles tendinopathy: comparison of flexor hallucis longus (FHL) transfer versus V-Y advancement. Int Orthop. 2018 Apr;42(4):829-34.
-
61Duthon VB, Lubbeke A, Duc SR, Stern R, Assal M. Noninsertional Achilles tendinopathy treated with gastrocnemius lengthening. Foot Ankle Int. 2011;32:375-9.
-
62Kiewiet NJ, Holthusen SM, Bohay DR, Anderson JG. Gastrocnemius recession for chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2013;34:481-5.
-
63Shakked RJ, Raikin SM. Insertional Tendinopathy of the Achilles Debridement, Primary Repair, and When to Augment. Foot Ankle Clin North Am. 2017;22:761-80.
-
64Kenneth J, Cohen BE, Davis WH, Anderson RB, Jones CP. Surgical Treatment of Insertional Achilles Tendinopathy With or Without Flexor Hallucis Longus Tendon Transfer: A Prospective, Randomized Study. Foot Ankle Int. 2015;36(9):998-1005.
-
65López-Capdevila L, Santamaría Fumas A, Domínguez Sevilla A, et al. Osteotomía calcánea con cuña de sustracción dorsal como tratamiento quirúrgico en la tendinopatía insercional de Aquiles. Rev Esp Cir Ortop Traumatol. 2020;64(1):22-7.
-
66Mafulli N, Gougoulias N, D’Addona A, Oliva F, Maffulli GD. Modified Zadek Osteotomy without excision of the intratendinous calcific deposit is effective for the surgical treatment of calcific insertional Achilles tendinopathy. Surgeon. 2020 Oct 22:S1479-666X(20)30143-8.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Tendon injury: from eternally forgotten to trendy disease
- Physiology and mechanobiology of tendon and muscle tissue
- Patellar tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Pathophysiology, diagnosis and treatment of Achilles tendinopathy
- Supraspinatus tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Results of arthroscopic gluteus medius tendon repair in patients with trochanteric pain syndrome. Case series
- Update on the diagnosis and treatment of quadriceps muscle injuries
- Management of triceps surae muscle injuries in young adult and middle-aged athletes: a narrative literature review
- Insertional tendinopathy of the Achilles tendon. Treatment from start to finish
- Inverted cyclops
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.