Results of arthroscopic gluteus medius tendon repair in patients with trochanteric pain syndrome. Case series
Resultados de reparación artroscópica de tendón glúteo medio en pacientes afectos de síndrome de dolor trocantérico. Serie de casos
Resumen:
Objetivos: evaluar los resultados de la reparación artroscópica del tendón glúteo medio en pacientes con síndrome de dolor trocantérico asociado a rotura de tendón del glúteo medio, con un seguimiento postoperatorio a 24 meses y revisión del estado actual del manejo de las mismas.
Material y métodos: estudio de cohorte retrospectiva. Se analizaron los resultados clínicos a 24 meses de 14 pacientes sometidos a reinserción artroscópica del glúteo medio. Los pacientes cumplimentaron cuestionarios funcionales, de calidad de vida y escalas de dolor en el preoperatorio y a los 24 meses postoperatorios.
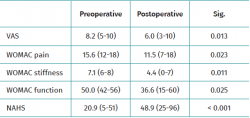
Resultados: 14 pacientes intervenidos por lesión de glúteo medio entre octubre de 2015 y marzo de 2019 fueron incluidos en esta serie. El dolor analizado con la escala visual analógica (VAS) mejoró de 8,2 (rango: 5-10) a 6,0 (rango: 3-10) a los 24 meses (p = 0,013). La evolución medida mediante la escala WOMAC, para el dolor pasó de 15,6 (rango: 12-18) preoperatorio a 11,5 (rango: 7-18) a 24 meses (p = 0,023); para rigidez, pasó de 7,1 (rango: 6-8) preoperatorio a 4,4 (rango: 0-7) a los 24 meses (p = 0,011); y para función, de 50,0 (rango: 42-56) preoperatorio a 36,6 (rango: 15-60) a los 24 meses (p = 0,025). La función, analizada con el Non-Arthritic Hip Score (NAHS), preoperatoria fue de 20,9 (rango: 5-51) y a los 24 meses de 48,9 (rango: 25-96) (p < 0,001).
Conclusión: la reparación artroscópica en pacientes afectos de síndrome de dolor trocantérico y con lesión del tendón del glúteo medio es una opción terapéutica que demuestra resultados favorables pero discretos a los 24 meses de la cirugía.
Abstract:
Objectives: to evaluate the results of arthroscopic repair of the gluteus medius tendon in patients with trochanteric pain syndrome associated to rupture of the gluteus medius tendon over a follow-up period of 24 months, and to conduct a review of the state of the art in the management of these disorders.
Material and methods: a retrospective cohort study was carried out. An analysis was made of the 24-month clinical outcomes of 14 patients subjected to arthroscopic reinsertion of the gluteus medius. The patients completed functional and quality of life questionnaires and pain scales preoperatively and 24 months after surgery.
Results: 14 patients operated upon due to gluteus medius injury between October 2015 and March 2019 were included in the present series. Pain as assessed by the visual analogue scale (VAS) improved from 8.2 (range 5-10) to 6.0 (range 3-10) after 24 months (p = 0.013). The WOMAC score for pain decreased from 15.6 (range 12-18) preoperatively to 11.5 (range 7-18) at 24 months (p = 0.023), while the stiffness score decreased from 7.1 (range 6-8) preoperatively to 4.4 (range 0-7) at 24 months (p = 0.011), and the function score decreased from 50.0 (range 42-56) preoperatively to 36.6 (range 15-60) after 24 months (p = 0.025). The function score as analysed by the Non-Arthritic Hip Score (NAHS) was 20.9 (range 5-51) preoperatively versus 48.9 at 24 months (range 25-96) (p < 0.001).
Conclusion: arthroscopic repair in patients with trochanteric pain syndrome and gluteus medius tendon injury is a therapeutic option that affords favourable results through discrete outcomes 24 months after surgery.
Introduction
Enthesopathy of the tendons of the gluteus medius and minimus, and the associated bursitis, are the most common causes of pain in the trochanteric space(1), and are encompassed within the so-called greater trochanter pain syndrome (GTPS) - a term introduced by Karpinsky and Piggott in 1985.
This clinical condition is highly prevalent and affects 17% of the population between 50-79 years of age(2). It is usually resolved based on conventional physiotherapy, physical postural correction measures and/or anti-inflammatory drugs or analgesics(3). However, between 30-40% of all cases (depending on the series) experience relapse over the short or middle term, or fail to respond to initial treatment – thus giving rise to chronification of the disorder(4,5). The affected patients experience a loss of quality of life similar to that seen in advanced-stage osteoarthrosis of the hip(6), and the disorder is difficult to resolve in a significant proportion of cases(7). In this respect, many conservative and surgical treatment protocols have been described, with highly variable published results(3,8). The lack of prospective studies and clinical trials involving long follow-up periods makes it difficult to establish consistent and solid management protocols.
The objective of the present study is to analyse the outcomes of a series of patients with chronic GTPS subjected to arthroscopic gluteus medius repair following failure of the adopted conservative measures, and offers a review of the state of the art of the different therapeutic options.
Material and methods
A retrospective cohort study was made of patients with GTPS and a magnetic resonance imaging (MRI) diagnosis of partial or complete rupture of the tendon of the gluteus medius, subjected to arthroscopic repair by the same surgeon between October 2015 and December 2018.
Inclusion criteria: patients of either gender between 18-75 years of age, with trochanteric pain for over 6 months and with a visual analogue scale (VAS) pain score of >3, a lack of response to conservative management in the form of physiotherapy or local or oral anti-inflammatory treatment, and MRI findings indicating partial or total rupture of the gluteus medius tendon.
Exclusion criteria: a history of other orthopaedic conditions of the affected hip (infant-juvenile hip diseases, hip dysplasia, femoroacetabular impingement or hip arthritis), as well as a history of open or arthroscopic surgery of the hip, femoral osteosynthesis with antegrade endomedullary pinning, or implant surgery, and patients not within the specified age range. Patients yielding incoherent information in the self-administered questionnaires during follow-up – defined as radically distinct answers to the same question in different questionnaires (e.g., no pain on walking over an even surface in the Non-Arthritic Hip Score [NAHS] and maximum pain possible on walking over an even surface in the WOMAC) – were excluded from the study. We also excluded patients with incomplete data in the preoperative period or over the two-year follow-up period.
Epidemiological data such as patient age and gender data were collected. Pain was assessed based on the VAS score from 0-10, where 0 = no pain and 10 = worst pain imaginable. The WOMAC in turn was used to assess pain (0: no pain; 18: maximum pain), stiffness (0: no stiffness; 8: maximum stiffness) and function (0: full function; 60: worst function possible), and the NAHS was used to evaluate non-arthritic hip pain and function (0: worst result; 100: best result possible). These scales were applied during the clinical follow-up of the patients, before surgery and at 24 months.
A radiological study was made to discard coxarthrosis (Tonnis grade ≤1) and hip dysplasia (Wiberg angle >20), and MRI was used to confirm partial or complete rupture of the gluteus medius tendon.
Surgical technique
All patients were operated upon in supine decubitus on a skeletal traction table. The anterolateral and distal lateral ports were used in all patients, and other accessory peritrochanteric ports were employed as required in each case, to facilitate suture handling. Endoscopy of the lateral compartment was performed combining the 30° and 70° optics. The gluteus medius injury was identified following partial resection of the trochanteric bursa in complete ruptures and following longitudinal incision of the superficial tendon in partial lesions. Debridement of the inflammatory tissue and degenerated tendon was carried out until healthy tendon extremities were obtained. Then, the insertional footprint in the greater trochanter was debrided and freshened with a bone drill to obtain a bleeding bed. Tendon repair was carried out using one or two 5-mm harpoons (Stryker® ReelX).
After surgery, partial weight bearing with two crutches was indicated during 6 weeks. Subsequently, functional rehabilitation was started with gluteus medius strengthening exercises, as well as improvement of pelvic pivoting and toning of the paravertebral and abdominal muscles to stabilize the lumbopelvic axis.
Statistical analysis
A descriptive analysis was performed, with calculation of the mean and range for quantitative variables. Categorical variables were reported as absolute and relative frequencies. The pre- and postoperative results of the patients were compared using the two-tailed Student t-test for repeated measures. Statistical significance was considered at a level of 0.05. The SPSS statistical package (SPSS Inc., Chicago, IL, USA) was used throughout the analysis.
Results
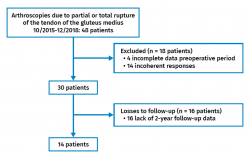
During the period between October 2015 and December 2018, a total of 48 patients underwent arthroscopic surgery for partial or complete rupture of the gluteus medius tendon, performed by the same surgeon. Four patients with incomplete data in the preoperative stage were excluded; 14 patients were excluded due to incoherent questionnaire responses; and 16 patients were excluded because they failed to answer some questionnaire over the two years of follow-up. A total of 14 patients that met the required criteria were thus finally analysed (Figure 1). Of these, 13 (92.9%) were women. The mean age was 67.3 years (range 48-76 years), with a body mass index (BMI) of 28.8 kg/m2 (range 22-34 kg/m2). Table 1 shows the results referred to pain as measured by the VAS, and pain, function and stiffness as assessed by the WOMAC and NAHS – significant improvement being observed at 24 months with all the scales.
Discussion
The patient flowchart (Figure 1) reflects a great percentage loss over follow-up (71% of the patients operated upon). The WOMAC and NAHS questionnaires were completed by the patients on an autonomous basis after receiving due explanations from the nursing staff. However, after analysing the questionnaires, many of them were found to have been completed incorrectly or only partially by the patients, and were therefore discarded. This is a controversial issue, since patient reported outcomes measures (PROM) must be completed by the patient, and counselling by the nursing staff could condition the responses. In order to avoid this, the questionnaires are given to the patients, an explanation is provided of how to complete them (cross in the right box, answer all the questions, etc.), and space is provided for the patients to complete the questionnaires on their own. In view of the many detected errors (fundamentally a high percentage of unanswered questions), which invalidated the questionnaire, a software system with an electronic tablet was introduced containing all the questionnaires and which did not allow the patients to advance further through the questions without first answering the preceding questions. This strategy allowed us to improve compliance with the questionnaires, but not the problem of incoherence due to failure to adequately understand the questions. The use of VISA-G has recently been proposed for the follow-up of patients with GTPS(9), seeking a questionnaire that is easier for them to understand. This questionnaire has not yet been validated in Spanish, however.
The results obtained reflect statistically significant improvement at 24 months. However, the clinical condition was not normalized, and residual pain, stiffness and functional impairment was documented in a large proportion of patients. These results are consistent with the different mentioned surgical options, affording very high improvement scores, but with frequent residual discomfort or pain. It must be taken into account that the patients included in our series had suffered different previous treatment failures, and the particularly high baseline scores conditioned the expected outcomes(10).
The calculation of minimal clinically important improvement (MCII) is of interest in this population. The study published by Okoroha establishes the MCII and PASS (patient acceptable symptomatic state) values for the Hip Outcome Score (HOS) and the modified Harris scale, but not for the WOMAC, VAS or NAHS(11). The mentioned study obtained a value above the PASS in 50-51% of the patients with the mentioned scales. In that study, the mean patient age was 57 years, 10 years younger than in our series, with a female prevalence and BMI that proved similar in both series. However, in our study the VAS score corresponding to preoperative pain was 8.2 versus 6.7 in the study of Okoroha - thus suggesting that patients in poorer preoperative condition or more chronic cases can be expected to yield poorer outcomes. Other series, such as that published by Byrd, with good outcomes in 12 patients subjected to two years of follow-up, also reflect a younger age than in our series, with an average of 56 years(12). The study carried out by McCormick, in a 65-year-old population, reported good or excellent outcomes in 60% of the patients, with the observation of a significant difference between the 60-year-old age group and the patients aged 70 years, in favour of the former(13).
In turn, the study carried out by Tubach et al. in an osteoarthritis population with less baseline pain according to the scales used (pain VAS score 5.6 and WOMAC score 58), concluded that the MCII was 2 for the VAS (0-10) and 9 for the WOMAC (0-100)(14). It may be expected that for the population in our study the MCII values would be higher, since the starting scores were higher. It possibly would be more interesting to obtain the PASS score, the value of which was 3.5 for the pain VAS and 34.4 for the WOMAC in the study of Tubach et al(15). The mentioned study involved patients with hip arthritis – not individuals with GTPS. However, studies such as that published by Ebert et al.(16) report similar scores on the pain and function scales in the osteoarthritis population and in the population diagnosed with chronic GTPS; the PASS values therefore could be extrapolated to the present study sample. Considering these values, it was seen that all the patients started from VAS and WOMAC scores above the PASS, and that after 24 months 29% and 43% yielded VAS and WOMAC results with values acceptable to the patient. It therefore could be concluded that, although improvement was observed, only 30-40% of the patients experienced improvement of a sufficient degree to afford acceptable outcomes.
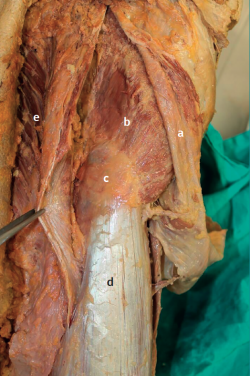
Anatomically, the hip adductors group is composed of the gluteus medius, the gluteus minimus with direct insertion in the proximal femur, the tensor fascia lata, and the most cranial portion of the gluteus maximus with insertion in the fascia lata (Figure 2). According to Gottschalk et al.(17), the tensor fascia lata muscle and the anterior portion of the gluteus medius are the main elements in charge of abduction of the hip, while the middle and posterior portions of the gluteus medius, as well as the gluteus minimus, act as hip stabilizers and pelvic rotators during walking. The innervation of each portion of the gluteus medius and of the tensor fascia lata muscle corresponds to branches of the superior gluteal nerve, penetrating from the internal surface for the gluteus medius and tensor, and from the superficial surface for the gluteus minimus. The gluteus medius consists of three portions: anterior, middle and posterior. The tendon of the gluteus medius inserts in the posterolateral part of the greater trochanter – the insertion of the posterior portion being the thickest. This appears to favour the fact that ruptures take place at its weakest part, which in turn participates in hip abduction along with the tensor fascia lata muscle(18). The gluteus minimus is located immediately beneath the gluteus medius and inserts anterior to the latter, as well as in the lateral capsule. Its fibres are distributed in parallel to the femoral neck, which in combination with the posterior portion of the gluteus medius defines it as a hip stabilizer(17).
Up to 7 bursae have been described in the peritrochanteric region. The term trochanteric bursa refers to the bursa beneath the gluteus maximus, which is located within the virtual space formed by the greater trochanter, the insertion and the muscle belly of the gluteus maximus, and the iliotibial tract. Only 20% of all peritrochanteric syndromes involve bursitis.
Disease associated to tendon degeneration, specifically of the gluteus medius and to a lesser extent of the gluteus minimus, is more prevalent in women (proportion 5:1), and is particularly seen in individuals between 40-60 years of age(19).
Other causes of pain in the trochanteric zone are stress fractures, heterotopic ossification, coxarthrosis and adaptive disorders such as residual dysplasia in the adult (RDA) and lumbar problems, with malfunction of the gluteus medius and secondary enthesopathy.
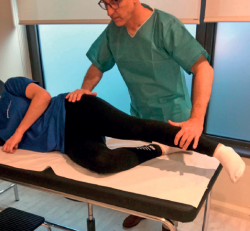
The physical examination of patients with GTPS should include the evaluation of gait in order to detect abductor muscle insufficiency (Trendelenburg gait), as well as the presence of this sign in sustained unipedal standing. Abduction against resistance in patients in lateral decubitus with the hip in neutral position and the knee in extension might not be painful, since the tensor fascia lata muscle contributes to execution of the movement. To neutralise it, we must evaluate pain in the greater trochanter on hip abduction with the knee in flexion and slight internal rotation. The test is considered to be positive when the patient experiences pain in this context. The above can be complemented with the Ober test, where passive adduction in that position generates pain due to tension of the fascia lata. A positive FABER manoeuvre helps to differentiate from hip joint disease.
In the abduction lag sign, the examiner passively moves the leg to abduction of about 30° in lateral decubitus and lets it drop suddenly – the patient being unable to keep the leg raised, which drops more than 10 cm (Figure 3).
A number of imaging techniques can help to confirm the diagnosis. Conventional radiology and computed tomography (CT) provide information on the biomechanical context and should always be considered, though they are of little use in assessing the condition of the tendon. Ultrasound can be very useful for evaluating the condition of the tendon and muscle, and for performing diagnostic punctures, though magnetic resonance imaging (MRI) is the technique of choice for diagnosing this particular disease condition, since it allows us to classify the degree of muscle atrophy and tendon retraction.
A number of therapeutic options are available, though there are no studies with a sufficient level of evidence to define a gold standard or establish a generalizable therapeutic recommendation.
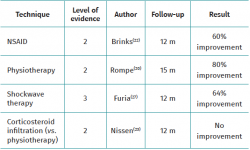
Table 2 describes the conservative management options.
Physiotherapy, weight loss and the use of non-steroidal anti-inflammatory drugs (NSAIDs) offer success rates of between 40-83%(8,20,21). With regard to the prescription of physical exercise, isometric exercises have been shown to be more effective than dynamic exercises in strengthening the gluteus medius and minimus. Unipedal standing and isometric abduction appear to ensure the greatest activity in this particular muscle group(4).
Corticosteroid infiltration with local anaesthetic is a very controversial practice(22). Despite a lack of clear supporting clinical evidence, this treatment is very widespread and standardised(23), since it does afford a short-term solution to the pain(8,20,22,23,24). However, this practice is not without side effects such as subcutaneous tissue atrophy, skin pigmentation changes and even tendon rupture(25). Serial infiltrations ultimately affect tendon quality, worsening the associated enthesopathy and complicating surgical repair(26). Ultrasound guided puncture affords success rates higher than puncture guided by clinical palpation (92% versus 45%) and may be considered for further defining the diagnosis. In addition, it can ensure peritendon infiltration and avoid intratendon injection, more clearly associated to tendon rupture(25). Recent clinical trials on the efficacy of local corticosteroids versus placebo have evidenced their inefficiency at three and 6 months(23), though such treatment is still used on a routine basis in clinical practice. In the present series, all of the patients had received corticosteroid infiltrations before surgery, though this variable was not included in the analysis because a very large number of subjects was unable to recall how many infiltrations they had received or whether they had received more than three infiltrations. This to some extent could affect the outcomes of tendon repair, in view of the even more devitalized condition of the tissue.
Shockwave therapy is based on the concentration of energy in the form of sound waves within the diseased tissues, with the purpose of promoting the repair processes. This principle underlies its use in chronic tendinopathy and enthesopathy. Furia et al. reported a good response to treatment with three shockwave sessions in a case-control study, documenting excellent or good outcomes in almost 80% of the cases in the shockwave group versus 36% in the control group(27). Rompe et al., in a randomised study of 249 patients, recorded significant superiority of shockwave therapy versus exercise combined with infiltrations(20). The effectiveness of the infiltrations was greater in the first month, but shockwave therapy demonstrated better results at four and 15 months. The current evidence suggests that shockwaves are effective at one year of follow-up, and therefore should be considered within the therapeutic scheme when physiotherapy and postural correction measures have failed(28). In our setting, shockwave therapy is not a routine practice, since not all physiotherapy centres assisting our reference population have such equipment; this parameter was therefore not included in the analysis. The use of this technique would be very interesting to determine whether those patients that improve as a result of surgery might also have improved with shockwave therapy, thereby rendering surgery unnecessary.
The administration of platelet-rich plasma (PRP) infiltrations has shown very erratic results; their use is therefore controversial. Jacobson et al. demonstrated that both ultrasound-guided fenestration and PRP infiltration are effective in treating gluteal tendinosis, with a percentage treatment response of 71% in the case of fenestration versus 79% with PRP at three months - no significant differences being observed between the two techniques(29). A randomised study has reported significant improvement versus treatment with corticosteroids(30), though this was a three-month study including patients with tendinopathy but without tendon rupture. Other randomised studies are ongoing(31), and it will be interesting to see their results. At present, the scarce evidence available demands caution in protocolising the use of this treatment modality.
The use of radiotherapy in the management on non-oncological disease has been well established(32). Low intensity radiotherapy has been proposed as a treatment option for GTPS associated to tendinopathy of the tendon of the gluteus medius(33), and the clinical series describe good outcomes(34) – though here again there is a lack of follow-up over the middle or long term. In our opinion, this is an attractive alternative, though long term evaluation is needed to confirm the good results and discard possible associated complications.
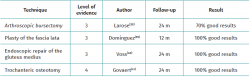
The surgical options yield highly variable results between different studies. Variability of the surgical indication criteria and the lack of universally accepted specific questionnaires and of a rigorous classification of the tendon lesion complicate evaluation of the different techniques, according to the review published by Ebert et al.(35). Table 3 describes the main surgical treatment options and their results.
It is surprising how such distinct surgical techniques (osteotomies, tendon repair procedures, bursectomy, plasty of the fascia lata, etc.) achieve such good outcomes, with different series reporting clinical or functional improvement rates of 100%. Nevertheless, the lack of middle or long term follow-up remains a constant factor.
According to Ebert et al., the outcome following open fixation of the tendons of the gluteus medius with augmentation plasty is independent of the degree of muscle atrophy prior to surgery. The authors concluded that the outcome of repair is not related to the degree of muscle atrophy before the operation but to the size of the defect and the technical capacity to repair it(36). However, Sonnery-Cottet et al., in their series of 22 cases, did find a relationship between the degree of atrophy and the outcomes of arthroscopic repair without augmentation(37).
One of the main limitations of our study is the limited sample size involved, which can be due to two main reasons. Firstly, these are complex patients in which the surgical decision is usually much delimited. Secondly, the techniques involved are evolving; it is therefore not possible to open a large recruitment window and maintain homogeneity of the surgical technique and criteria. The results of the series at one year of follow-up are presented, when in fact a minimum follow-up of two years should be maintained in a surgical series.
Lastly, a factor associated to poorer outcomes is the presence of degenerative disc disease, which was not contemplated in our series and may affect the outcomes in an indeterminate manner.
Conclusion
The treatment of GTPS with associated partial or complete gluteus medius tendon rupture via arthroscopic repair affords good results after 24 months of follow-up in terms of pain, function and stiffness of the hip in our clinical series.
In our clinical series, the disease was more prevalent in women in the sixth to seventh decades of life.
The literature describes a broad range of conservative and surgical treatment options, with few clinical studies offering relevant results over the middle or long term.
Physiotherapy, changes in lifestyle and shockwave therapy should be considered in the GTPS management protocols. Such consideration does not apply to corticosteroid infiltrations, which should be limited and viewed with caution.
Tablas
Figuras
Figure 2. View of the tendon of the gluteus medius beneath the tensor fascia lata after opening the Gibson interval. a: fascia lata; b: gluteus medius; c: tip of the greater trochanter; d: vastus lateralis; e: gluteus maximus.
Información del artículo
Cita bibliográfica
Autores
Marc Tey Pons
Unidad de Cadera. iMove Traumatología. Hospital Mi Tres Torres. Barcelona
I-Move Traumatologia. Barcelona
Hospital Universitari Parc Taulí. Sabadell
Grupo Ibérico de Preservación de Cadera (GIPCA)
Universidad Pompeu Fabra
Unidad de Cadera. Servicio de Cirugía Ortopédica y Traumatología. Hospital del Mar. Barcelona
Unidad de Artroscopia y Rodilla. ICATME-Institut Universitari Dexeus. Universitat Autònoma de Barcelona
Alfonso Alías Petralanda
Hospital Clínic. Barcelona
Oliver Marín Peña
Unidad de Cadera. Hospital Universitario Infanta Leonor. Madrid
Servicio de Cirugía Ortopédica y Traumatología. Hospital Universitario Infanta Leonor. Madrid
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved human or animal experimentation.
Data confidentiality. The authors declare that the protocols of their work centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Kingzett-Taylor A, Tirman PF, Feller J, et al. Tendinosis and tears of gluteus medius and minimus muscles as a cause of hip pain: MR imaging findings. Am J Roentgenol. 1999 Oct;173(4):1123-6.
-
2Segal NA, Felson DT, Torner JC, et al. Greater Trochanteric Pain Syndrome: Epidemiology and Associated Factors. Arch Phys Med Rehabil. 2007 Aug;88(8):988-92.
-
3Reid D. The management of greater trochanteric pain syndrome: A systematic literature review. J Orthop. 2016 Mar;13(1):15-28.
-
4Lievense A, Bierma-Zeinstra S, Schouten B, Bohnen A, Verhaar J, Koes B. Prognosis of trochanteric pain in primary care. Br J Gen Pract. 2005 Mar;55(512):199-204.
-
5Fearon AM, Scarvell JM, Cook JL, Smith PNF. Does ultrasound correlate with surgical or histologic findings in greater trochanteric pain syndrome? A pilot study. Clin Orthop Relat Res. 2010;468(7):1838-44.
-
6Fearon AM, Cook JL, Scarvell JM, Neeman T, Cormick W, Smith PN. Greater Trochanteric Pain Syndrome Negatively Affects Work, Physical Activity and Quality of Life: A Case Control Study. J Arthroplasty. 2014;29(2):383-6.
-
7Fearon AM, Cook JL, Scarvell JM, Neeman T, Cormick W, Smith PN. Greater trochanteric pain syndrome negatively affects work, physical activity and quality of life: a case control study. J Arthroplasty. 2014;29(2):383-6.
-
8Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: A systematic review. Br J Sports Med. 2017;51(2):97-104.
-
9Fearon AM, Ganderton C, Scarvell JM, et al. Development and validation of a VISA tendinopathy questionnaire for greater trochanteric pain syndrome, the VISA-G. Man Ther. 2015;20(6):805-13.
-
10Ornetti P, Dougados M, Paternotte S, Logeart I, Gossec L. Validation of a numerical rating scale to assess functional impairment in hip and knee osteoarthritis: comparison with the WOMAC function scale. Ann Rheum Dis. 2011;70(5):740-6.
-
11Okoroha KR, Beck EC, Nwachukwu BU, Kunze KN, Nho SJ. Defining Minimal Clinically Important Difference and Patient Acceptable Symptom State After Isolated Endoscopic Gluteus Medius Repair. Am J Sports Med. 2019 Nov 16;47(13):3141-7.
-
12Byrd JWT. Peritrochanteric Access and Gluteus Medius Repair. Arthrosc Tech. 2013;2(3):e243-6.
-
13McCormick F, Alpaugh K, Nwachukwu BU, Yanke AB, Martin SD. Endoscopic repair of full-thickness abductor tendon tears: surgical technique and outcome at minimum of 1-year follow-up. Arthroscopy. 2013 Dec;29(12):1941-7.
-
14Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29-33.
-
15Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: The patient acceptable symptom state. Ann Rheum Dis. 2005;64(1):34-7.
-
16Ebert JR, Retheesh T, Mutreja R, Janes GC. The Clinical, Functional and Biomechanical Presentation of Patients With Symptomatic Hip Abductor Tendon Tears. Int J Sports Phys Ther. 2016;11(5):725-37.
-
17Gottschalk F, Kourosh S, Leveau B. The functional anatomy of tensor fasciae latae and gluteus medius and minimus. J Anat. 1989;166:179-89.
-
18Tsutsumi M, Nimura A, Akita K. The gluteus medius tendon and its insertion sites: an anatomical study with possible implications for gluteus medius tears. J Bone Joint Surg Am. 2019;101(2):177-84.
-
19Fearon A, Neeman T, Smith P, Scarvell J, Cook J. Pain, not structural impairments may explain activity limitations in people with gluteal tendinopathy or hip osteoarthritis: a cross sectional study. Gait Posture. 2017;52:237-43.
-
20Rompe JD, Segal NA, Cacchio A, Furia JP, Morral A, Maffulli N. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanter pain syndrome. Am J Sports Med. 2009;37(10):1981-90.
-
21Lustenberger DP, Ng VY, Best TM, Ellis TJ. Efficacy of treatment of trochanteric bursitis: a systematic review. Cli J Sport Med. 2011 Sep;21(5):447-53.
-
22Brinks A, van Rijn RM, Willemsen SP, et al. Corticosteroid Injections for Greater Trochanteric Pain Syndrome: A Randomized Controlled Trial in Primary Care. Ann Fam Med. 2011 May 1;9(3):226-34.
-
23Nissen MJ, Brulhart L, Faundez A, Finckh A, Courvoisier DS, Genevay S. Glucocorticoid injections for greater trochanteric pain syndrome: a randomised double-blind placebo-controlled (GLUTEAL) trial. Clin Rheumatol. 2019 Mar 28;38(3):647-55.
-
24Torres A, Fernández-Fairen M, Sueiro-Fernández J. Greater trochanteric pain syndrome and gluteus medius and minimus tendinosis: nonsurgical treatment. Pain Manag. 2018;8(1):45-55.
-
25Shapiro PS, Rohde RS, Froimson MI, Lash RH, Postak P, Greenwald AS. The Effect of Local Corticosteroid or Ketorolac Exposure on Histologic and Biomechanical Properties of Rabbit Tendon and Cartilage. Hand. 2007 Dec 5;2(4):165-72.
-
26Fadale PD, Wiggins ME. Corticosteroid Injections: Their Use and Abuse. J Am Acad Orthop Surg. 1994 May;2(3):133-40.
-
27Furia JP, Rompe JD, Maffulli N. Low-Energy Extracorporeal Shock Wave Therapy as a Treatment for Greater Trochanteric Pain Syndrome. Am J Sports Med. 2009 Sep 13;37(9):1806-13.
-
28Mani-Babu S, Morrissey D, Waugh C, Screen H, Barton C. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: a systematic review. Am J Sports Med. 2015;43(3):752-61.
-
29Jacobson JA, Yablon CM, Henning PT, et al. Greater trochanteric pain syndrome: percutaneous tendon fenestration versus platelet-rich plasma injection for treatment of gluteal tendinosis. J Ultrasound Med. 2016 Nov;35(11):2413-20.
-
30Fitzpatrick J, O’Donnell J. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: Response. Am J Sports Med. 2018;46(8):NP33-4.
-
31Oderuth E, Ali M, Atchia I, Malviya A. A double blind randomised control trial investigating the efficacy of platelet rich plasma versus placebo for the treatment of greater trochanteric pain syndrome (the HIPPO trial): a protocol for a randomised clinical trial. Trials. 2018;19(1):1-9.
-
32Ott OJ, Niewald M, Ingrid HW, et al. DEGRO guidelines for the radiotherapy of non-malignant disorders Part II: Painful degenerative skeletal disorders. Strahlenther Onkol. 2015 Jan;191(1):1-6.
-
33Valduvieco I, Biete A, Moreno LA, et al. Is anti-inflammatory radiotherapy an effective treatment in trochanteritis? Br J Radiol. 2017;90(1069):20160520.
-
34Kaltenborn A, Martin U, Tanja C, Mirko H, Hermann RM. Low-dose external beam radiotherapy for greater trochanteric pain syndrome: Target volume definition and treatment outcome. Strahlenther Onkol. 2017 Apr;193(4):260-8.
-
35Ebert JR, Bucher TA, Ball SV, Janes GC. A review of surgical repair methods and patient outcomes for gluteal tendon tears. Hip Int. 2015;25(1):15-23.
-
36Ebert JR, Smith A, Breidahl W, Fallon M, Janes GC. Association of Preoperative Gluteal Muscle Fatty Infiltration With Patient Outcomes in Women After Hip Abductor Tendon Repair Augmented With LARS. Am J Sports Med. 2019 Nov;47(13):3148-57.
-
37Thaunat M, Clowez G, Desseaux A, et al. Influence of Muscle Fatty Degeneration on Functional Outcomes After Endoscopic Gluteus Medius Repair. Arthroscopy. 2018 Jun;34(6):1816-24.
-
38Larose C, Guanche CA. Paper 28: Arthroscopic Treatment of Recalcitrant Greater Trochanteric Bursitis with Minimum Two-Year Followup. Arthroscopy. 2012 Jun;28(6):e59-60.
-
39Domínguez A, Seijas R, Ares O, Sallent A, Cuscó X, Cugat R. Clinical outcomes of trochanteric syndrome endoscopically treated. Arch Orthop Trauma Surg. 2015;135(1):89-94.
-
40Monllau JC, Leal J, Voss C, Pelfort X, Tey M, Pavlovich RI. Good outcome after meniscal repair using an all-inside suturing system in combination with high-frequency biostimulation. Orthopedics. 2010;33(6).
-
41Govaert LHM, van der Vis HM, Marti RK, Albers GHR. Trochanteric reduction osteotomy as a treatment for refractory trochanteric bursitis. J Bone Joint Surg Br. 2003 Mar;85(2):199-203.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Tendon injury: from eternally forgotten to trendy disease
- Physiology and mechanobiology of tendon and muscle tissue
- Patellar tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Pathophysiology, diagnosis and treatment of Achilles tendinopathy
- Supraspinatus tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Results of arthroscopic gluteus medius tendon repair in patients with trochanteric pain syndrome. Case series
- Update on the diagnosis and treatment of quadriceps muscle injuries
- Management of triceps surae muscle injuries in young adult and middle-aged athletes: a narrative literature review
- Insertional tendinopathy of the Achilles tendon. Treatment from start to finish
- Inverted cyclops
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.