Physiology and mechanobiology of tendon and muscle tissue
Fisiología y mecanobiología del tejido tendinoso y muscular
Resumen:
El complejo estructural que forma la unidad tendón-músculo debe ser ampliamente conocido para proporcionar un correcto diagnóstico y tratamiento a los pacientes que sufren estas lesiones.
Los tendones funcionan principalmente como elementos de transmisión de carga (tensión) de músculo a hueso. Son estructuras dinámicas que responden a la frecuencia, la magnitud, la duración y la dirección de los estímulos mecánicos o cargas. La respuesta tiene lugar a través de interacciones complejas entre las células y la matriz extracelular, que es una estructura altamente especializada.
El músculo, además de proporcionar andamiaje estructural al organismo, es el responsable de la movilidad articular mediante la contracción, permitiendo actividades complejas como andar, saltar o correr.
El objetivo de este artículo es exponer el conocimiento actual sobre la fisiología y la mecanobiología tanto del tendón como del músculo y analizar desde su arquitectura la respuesta a estímulos mecánicos, en un enfoque mecanobiológico respecto a su función.
Abstract:
The structural complex formed by the tendon-muscle junction must be widely well known in order to allow the correct diagnosis and treatment of patients with musculotendinous injuries.
Tendons mainly act as load (tension) transmission elements between muscle and bone. They are dynamic structures that respond to the frequency, magnitude, duration and direction of the mechanical stimuli or loads. This response is mediated by complex interactions between the cells and the extracellular matrix, which is a highly specialised structure.
On the other hand, the muscle affords structural scaffolding for the body, and is moreover responsible for joint mobility through contraction, -allowing complex activities such as walking, jumping or running.
The present study describes current knowledge of the physiology and mechanobiology of both tendons and muscles, and analyses their response to mechanical stimuli on the basis of their architectural features, adopting a mechanobiological approach.
Introduction
It is essential to know the physiology and mechanobiology of tendon and muscle tissue in order to provide optimum treatment for the disease conditions found in sports traumatology. The complexity of the architecture and histology, associated to innervation and neurotransmission, are important in order to understand the mechanobiology and mechanotransduction of tendon cells and muscle cells. This conditions optimisation of the functioning of these tissues when they are healthy, and the ability to analyse the pathophysiology underlying injuries, with a view to ensuring adequate patient recovery.
The present study describes current knowledge of the physiology and mechanobiology of both tendons and muscles. In addition, it analyses from their architectural features their response to mechanical stimuli, adopting a mechanobiological approach to the function of these tissues.
Tendons
Tendons are dynamic structures that respond to physiological or pathological mechanical loads through complex interactions between their cellular components and the extracellular matrix (ECM). Mechanobiology and mechanotransduction describe this response(1).
Architecture and histology
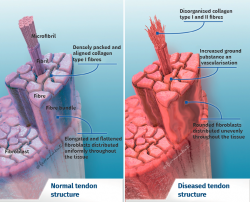
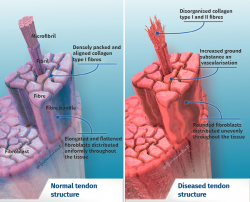
Tendon architecture is characterised by a hierarchical fibrillar organisation with a sequence of collagen molecules that form fibrils, fibres and fascicles (bundles of fibres). All this in turn is surrounded by a fibrous layer known as the epitenon, which reduces friction with the surroundings. Collagen represents 70-80% of the dry weight of a normal tendon. There are many different forms of collagen (types I, III, V, IX, X, XI, XII), with different structural, mechanical and reparative functions(1). In addition, tendons contain proteoglycans such as decorin and aggrecan (which maintain hydration and facilitate sliding between molecules), as well as glycoproteins such as tenascin C, fibronectin and elastin (which maintain mechanical stability and facilitate the return to resting condition after biological loading) — all organised in alignment with the longitudinal axis of the tendon (Figure 1).
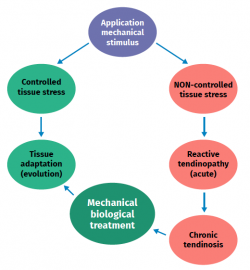
The cellular composition in turn comprises different types of cells: tenocytes, chondrocytes, synovial cells, vascular cells and stem cells. Tendon cells are interspaced among the collagen fibres and organised into a network aligned along the major axis of a tendon. Tenocytes constitute the most abundant cell population and are in charge of producing ECM, maintaining tendon homeostasis and repairing injuries. These are flattened cells that are in contact with the neighbouring cells through somatic prolongations, and are in direct contact with the collagen bundles. The somatic prolongations in turn are interconnected through gap junctions and with the ECM(1,2). They are sensitive to mechanical stimuli and adapt the ECM through anabolic or catabolic changes according to the magnitude, frequency, direction and duration of the externally applied loads(3,4,5). This interaction is mediated by a series of pathways between the cell surface (such as ion channels, integrins, the cellular cytoskeleton and kinases) and the nucleus of the cells, producing a biological response. Physiological loads are necessary in order to maintain tendon homeostasis. In contrast, abnormally high loads can cause an injury in the form of acute trauma or chronically secondary to the accumulation of damage arising from an altered cellular-matrix response(2,6).
The other tendon cells are less frequent and are found in special locations. Thus, chondrocytes are present at the tendon-bone junction; synovial cells are found in the paratenon; and vascular cells are located in the nurturing vessels of the tendon and in pathophysiological form in situations of hypervascularisation. Stem cells are also important, and play key roles in repair, though in the pathophysiology of tendinopathic conditions they have been reported to give rise to aberrant differentiations(7,8).
Innervation and neurotransmission
Although tendon innervation is defined to be poor in comparison with muscle innervation, it also influences the biological response of the tendon and is essential in the regeneration of tendon injuries(9).
This innervation originates from the myotendinous junction and the paratenon. The nerve endings can be classified according to their functions as follows(10):
a) Mechanoceptors:
a.1) Type I (Ruffini), sensitive to stretching and pressure.
a.2) Type II (Vater-Pacini), sensitive to changes in pressure and velocity (elongation, acceleration and deceleration).
a.3) Type III (Golgi), sensitive to changes in tension.
b) Nociceptors (type IVa fibres).
c) Autonomic system (type IVb fibres), located mainly in the wall of small blood vessels.
In addition to the afferent functions that can be deduced from its description (mechanoreception, nociception and vasomotor modulation), this nervous system has efferent functions in cell proliferation, in the expression of cytokines and growth factors, in inflammation, in the immune response, and in the paradoxical efference of the nociceptive afferent fibres described by Bayliss in 1901(11). Efferent action is mediated by classical neurotransmitters (monoamines, acetylcholine) and neuropeptides. Both act as chemical messengers and are stored in the nerves fibres in vesicles of different sizes; depending on the size of the vesicles, they respond to different action potential stimulation frequencies. Tendon homeostasis is largely dependent upon the neuromediating balance originating from the periphery of the tendon body(10). The balance in which classical neuropeptides and neurotransmitters exert effects upon the proliferation process and the inflammation process participates in regeneration. Following lesion onset, the described nerve endings expand from the periphery towards the body of the tendon, concomitantly to vascular growth and migration of inflammatory cells, and they subsequently retract once the tissue has been repaired.
Mechanobiology and mechanotransduction
Knowing the mechanobiology of the tendon cells is crucial in order to understand both the benefits of controlled loads upon tendon tissue and the pathophysiology of tendon injury. Mechanical loads are transmitted to the tendon cell through the ECM. Such transmission generates transduction phenomena in the cell through transmembrane structures that initiate the biochemical response. Deformation of the ECM of the tendon transmits traction, compression and shearing stress to the cells. In addition, within the tendon itself, interstitial fluid transfer also takes place, apparently playing a role in stimulation of the cells. The cellular transduction phenomena are mediated by multiple mechanisms and pathways, including the primary cilium (present in many mammalian cells), membrane receptors, ion channels, ATP activation, cytoskeletal changes, modifications in gene expression, etc. Tendon cells react to loading forces by activating the calcium channels, which increases the concentration of Ca++, resulting in ATP release, changes in the expression of certain cytoplasmic filaments (e.g., actin) and an increased secretion of extracellular matrix metalloproteinases (MMP)(12,13,14).
In the case of the tenocytes, there is growing evidence that their elasticity / rigidity is very important in their relationship with the ECM. Tenocyte deformability depends on the cytoskeleton (basal tension level), which in turn depends on the tension status of the ECM itself and on the connections of the cell with the ECM (integrins) and with other cells. Tenocyte response to tendon loading (of the ECM) is largely dependent upon the basal tension level of the cytoskeleton. Likewise, there are biochemical mediators that regulate the mechanical properties of the tenocyte and of the surrounding matrix, and which therefore modulate its mechanosensitivity(15). The ECM transduces the external deformation to the cell nucleus through integrin proteins and the cytoskeleton(16). The primary cilium is oriented longitudinally (parallel) to the collagen fibres, and it appears to play an important role in this entire homeostasis process. Its length has been seen to increase in situations of load privation — an effect that can be reverted by reintroducing the effect of mechanical loading(17).
There are also connections between the cells of the epitenon and the tenocytes through proteins known as connectins(1). These are dynamic structures that also exert mechanobiological activity upon the tenocyte.
The forces generated in the tendon (magnitude, frequency and duration) depend on prior tendon conditioning (history of previous loading: exercise, overuse, disuse) and the composition of the ECM — which can change with age, gender, previous disease or, simply, the type of tendon involved.
Excessive mechanical stimulation of the tendon through repeated loading can cause microscopic fibrillar damage (Figure 2)(18,19). At the same time, cell function inhibition occurs(6,20), with consequent alteration of the cell-matrix relationship with the following sequence:
- Collagen fibre rupture.
- Hypocellularity.
- Increase of the metalloprotease levels.
- Consequent apoptosis.
Excessive loading stimuli give rise to increased PGE2 production; this inflammatory mediator appears to influence differentiation of the stem cells into cells other than tenocytes (thus producing tissue different from tendon tissue)(8,21). On the other hand, when the loading stimulus is insufficient, changes are observed in the shape and number of tendon cells (mainly tenocytes), the amount of collagen and proteoglycans, and in the way in which the glycoproteins align (demonstrated in collagen type I, III, aggrecan, decorin and fibronectin). Furthermore, there are changes in the expression of MMP and of MMP inhibitors(1).
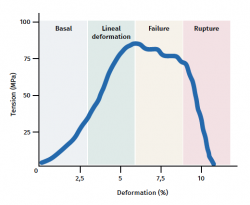
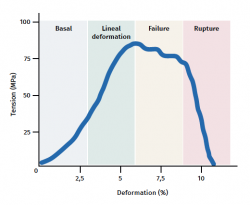
The tendon exhibits characteristic mechanical behaviour, and the load/deformation curve presents four phases (Figure 3):
a) Deformation less than 2% (stretching). Corresponds to disappearance of the typical wavy pattern of the tendon fibres. The response in this phase depends on the type and location of the tendon.
b) Deformation less than 4% (linear region). Represents the limit of the physiological deformation, and its slope is indicated by the elasticity modulus (Young's modulus).
c) Deformation between 4-8%. Corresponds to the appearance of microruptures.
d) Deformation over 8%. Corresponds to macroscopic ruptures or complete rupture of the tendon.
Lastly, the tendons, in the same way as all biological tissues, are viscoelastic, since fluid transfer in the ECM conditions the mechanical response. Therefore, deformation depends on the velocity of loading and increases with lower velocities, and vice versa. At high loading velocities, the tendon becomes stiffer and is more effective in transmitting the load to bone(22).
Treatment strategies
One of the major problems is that although an abnormal level of mechanical stimulation is known to induce pathological processes, the adequate loading level (magnitude, frequency, duration) for maintaining normal tendon homeostasis is not known, and the precise loading level to favour diseased tendon repair is likewise unclear.
One of the most widely used and effective treatments for managing tendinopathies in general is eccentric exercise (Figure 4). However, exactly how such loading upon the tendon counters alteration of the mechanobiological process originating the tendinopathy is not known(23). The other treatment alternatives are explained in relation to each of the tendinopathies commented below.
Muscles
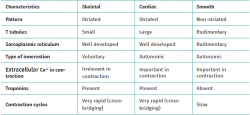
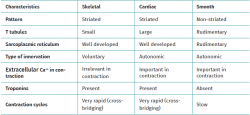
Muscle tissue is classified into different types (striated, smooth and cardiac), and each of them have a particular behaviour (Table 1). Striated musculature is responsible for voluntary motor function. The muscles represent between 40-45% of total body weight, and the muscle fibre is the basic macroscopic functional unit of muscle.
Force exerted against an external load generates tension, with the production of muscle contraction. If the force exceeds the load, muscle fibre shortening takes place (concentric phase), whereas if the load is not exceeded by the force, no contraction with muscle shortening occurs, resulting in isometric contraction. Eccentric contraction occurs during elongation of the muscle and has been shown to be very useful in the muscle and tendon regeneration process. In addition, eccentric contraction results in a greater increase in global strength and muscle mass compared with concentric work(24).
Architecture and histology
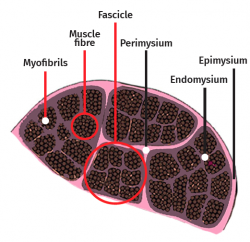
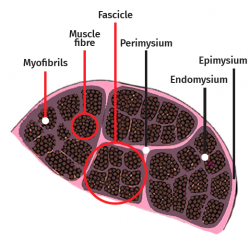
The skeletal muscle cell is multinucleated, cylindrical and possesses contractile capacity. Each cell is covered by connective tissue, forming an envelope known as the endomysium. The perimysium in turn is the connective covering enveloping a group of muscle cells, forming fascicles, connective tissue tunnels and intramuscular septae that traverse the muscle belly and provide an entry route for arterioles, venules and nerves. In turn, the entire muscle is enveloped by a thick layer known as the epimysium, which separates it from the surrounding muscles. These layers of connective tissue can continue with the fibrous tissue that forms the tendons, and proves to be essential for transmission of the force generated by the muscle cells to the skeletal structures(25) (Figure 5).
The cell membrane of the myocyte is called the sarcolemma, while the cytoplasm is referred to as the sarcoplasm. The latter contains numerous fine fibril bundles known as myofibrils, which in turn are composed of even thinner fibres called actin and myosin myofilaments. The myofibrils are divided into a series of longitudinally repeating units known as sarcomeres, that represent the functional units of striated muscle.
Myosin is a very complex motor protein or enzyme, since it belongs to the group of enzymes that convert ATP into ADP and phosphates (thus constituting an ATPase). This enzymatic activity generates traction forces with energy expenditure (ATP), with displacement along the actin filament towards the most polarised extremity.
Actin in turn is a globular protein that undergoes polymerization (binding of G-actin molecules) to conform the actin filament (F-actin). There are two types: alpha and beta. The alpha type is abundant in striated muscle, while the beta type is abundant in most animal cells. The filaments measure 7 mm in length, and are therefore also referred to as microfilaments. The mechanism underlying increasing and shortening of the actin filaments is mediated by polymerization; specifically, polymerization predominates at one end of the filament (addition of new G-actin molecules), while depolarization predominates at the other end.
The T tubules and sarcoplasmic reticulum are composed of a developed system of membranes present in striated muscle. The T tubules are prolongations of the sarcolemma, distributed transversal to its surface, and for this reason they are also known as transverse tubules. They are in charge of transmitting the electrical impulse towards the interior of the cell, specifically to the membrane of the sarcoplasmic reticulum.
The sarcoplasmic reticulum is an organelle similar to the endoplasmic reticulum, whose main function is protein production. However, in contrast to the latter, the sarcoplasmic reticulum is in charge of the intracellular calcium storage, regulating this concentration via the Ca++ ion pumps present in its membrane. This process uses ATP as fuel to absorb Ca++ towards the interior of the reticulum, giving rise to a process of relaxation-contraction of the striated muscle.
The satellite cell (so called because of its peripheral location) is found between the basal lamina, being surrounded by the latter, and the sarcolemma. The cell remains under resting conditions until an external stimulus (physical exercise) activates it(26). Once the satellite cells are activated, they are easily morphologically identifiable as a bulging in the muscle fibre, with the presence of cytoplasmic processes that extend towards one or both cell poles, reflecting the increase in their mitotic activity(25). Satellite cells intervene in the muscle regeneration process. The activity of the satellite cells, in response to different stimuli, is related to activation without proliferation, proliferation without posterior differentiation, or proliferation and differentiation (depending on the stimuli related to hypertrophy or regeneration processes)(25).
Innervation and neurotransmission
As commented above, stimulation from the motor nerve fibres results in arrival of the action potential along the axons of the motor nerves, with the release of acetylcholine at synaptic level, stimulating the release of Ca++ from the sarcoplasmic reticulum and thus stimulating the muscle fibre — with consequent excitation of the latter and the activation of muscle contraction(26,27).
Muscle fibres are innervated by ramifications of the axons of the motor nerve fibres (neurofibrils) originating from the spinal cord. The axon-muscle fibre complex is referred to as the motor unit.
There are three types of motor units:
- Type I (slow and fatigue-resistant): type I fibres are characterised by a high mitochondrial density and intense oxidative enzyme activity, accompanied by aerobic energy production, rich capillary vascularisation, high myoglobin concentration and low myosin-ATPase activity(28). They are able to generate discrete tension for prolonged periods of time without fatigue; in addition, the force they generate increases and decreases slowly. In these units, the motor neurons are of smaller size, with a slower conduction velocity and a lesser excitation threshold than the other types of fibres. They are characteristically associated to resistance sports (marathon, ultra trail and cycling).
- Type IIA (rapid and fatigue-resistant): these fibres combine properties of type I and IIX/IIB fibres, since they have sufficient aerobic capacity to resist fatigue during several minutes.
- Type IIX/IIB (rapid and fatigable): type IIX fibres are present in humans, while type IIB fibers are exclusively found in animal muscles. They benefit from anaerobic energy production and are characterised by a low mitochondrial density. Their main way of obtaining energy is through glycolysis. These fibres present high CK activity, poor capillary vascularisation, low myoglobin concentration and high myosin-ATPase activity(28). The muscle fibres are large and develop greater strength over short periods of time thanks to their anaerobic metabolism. The motor neurons are large, with high conduction velocities and excitation thresholds(29). They are characteristically associated to strength-related sports.
Mechanobiology and mechanotransduction
In the same way as applies to the tendon, and taking into account the close association to muscle, it is crucial to understand these events for better comprehension and application of the current evidence in the treatment of muscular or myotendinous lesions.
Mechanotransduction is the cellular deformation that occurs when the cell perceives and adapts to its surroundings through cytoskeletal changes that are transmitted to the nucleus. These changes range from a simple modification of cell shape to the expression of new proteins. Processes such as hyperplasia or dysplasia and remodelling are clear examples of this mechanism. This is a relatively new field of study that has made it possible to establish these relationships through multidisciplinary research, though we still need to more fully explore and integrate many of the implicated elements.
The muscle loading mode that comes quickest to mind is loading induced by the generation of active force. The adaptability of skeletal muscle to the changes in the mechanical environment has been well established at tissue and system level, though the mechanisms whereby the mechanical signals are transduced into chemical signals that in turn influence muscle growth and metabolism remain largely unclear. Nevertheless, a number of findings suggest that mechanical signal transduction in muscle may occur through signalling pathways that are shared with insulin-like growth factor I (IGF-I). The participation of signalling mediated by IGF-I in mechanical signal transduction in muscle was initially suggested from the following observations(30):
- The muscle releases IGF-I in mechanical stimulation.
- IGF-I is a potent promoter of muscle growth and affects the phenotype.
- IGF is able to function as an autocrine hormone in muscle.
The evidence shows that at least two signalling pathways resulting from the binding of IGF-I may influence muscle adaptation and growth. It has been shown that signalling through calcineurin / nuclear factor of the activated T cell pathway exerts a potent influence upon the promotion of the slow / type I phenotype in muscle, and can increase muscle mass. This pathway can be activated by neural stimulation of the muscle. It remains to be studied whether neural activation of the pathway is independent of mechanical activation or IGF-I mediated signalling(30).
General tension does not regulate muscle fibre size in a single manner. Force transmission through pathways different from the myotendinous junctions may contribute to the reported discrepancies, due to the substantial in series heterogeneity of sarcomere length within the muscle fibres that produce local variations in the mechanical stimuli for adaptation. In the case of the isolated muscle fibre, mechanical signalling is quite different to that seen in the in vivo or in vitro setting. The elimination of the traction and shearing effects of the adjacent tissues (even the antagonist muscle) modifies or suppresses the mechanical stimuli for adaptation(31).
The contractile apparatus is the most significant structure of a muscle fibre and can constitute an excellent cell deformation marker. The interdigital matrixes of thick and thin filaments are rigidly immobilised by the M and Z discs, respectively, and the thick filaments are anchored to the Z disc by titin. As the fibre elongates, the filaments slide against each other.
The sliding model proposes that the thin filaments slide over the thick filaments. Such displacement is possible thanks to the binding between the myosin heads and active or complementary points of the actin molecules. Binding through cross-bridging between actin and myosin that is cyclically activated and deactivated constitutes the process resulting in muscle shortening during contraction(27).
The stimulus received through the motor nerve fibres generates a muscle action potential that extends over the entire membrane or sarcolemma.
Muscle contraction results from sliding between the thin and thick filaments (actin and myosin II, respectively). Such interdigitation of the filaments produces a shortening of sarcomere length.
The maximum force generated by contraction is directly proportional to the cross-section of the muscle.
The distribution of the muscle fibres with respect to the longitudinal axis divides muscles into:
- Fusiform: the fibres run parallel to the longitudinal axis of the muscle.
- Penniform: the fibres form an angle with the longitudinal axis of the muscle.
Penniform muscles develop greater strength, since there are more cells in their section.
Types of muscle injury and classification
Muscle injuries are very common in sports. Specifically, in the case of soccer(32), rugby(33) or athletics(34), the recorded incidences exceed 30%.
The injuries are classified into indirect and direct, according to the hurtful mechanism involved. Direct muscle injuries are most often caused by contusions, while indirect injuries are particularly caused in the eccentric contraction phase(26).
The anatomical location of the injury in the muscle is as important as its extent, since the amount of affected connective tissue (myofascial, myotendinous, intramuscular) conditions the time to recovery and the return to sports activities(35,36). Biarticular muscles (biceps femoris, gastrocnemius and rectus femoris) are also more vulnerable to injury due to indirect mechanism in the eccentric phase, in view of their biomechanical characteristics, with tension increments that exceed muscle resistance. When the mechanism involves an increase in eccentric contraction, it is more frequent to observe injury at the myotendinous junction(26,27), together with fatigue and failure of the cellular regeneration and repair mechanisms after rupture.
The non-viable muscle fibres are eliminated by tissue macrophages, phospholipase A2 activation, an increase in proteases and prostaglandins, and a decrease in creatine kinase. Intracellular calcium accumulation occurs due to failure of the sarcoplasmic reticulum, with an increase in temperature and of free oxygen radical presence, and a consequent decrease in pH(37).
A dual repair response thus takes place:
- The satellite cells differentiate into myoblasts and fuse to form new muscle fibres (regeneration).
- A variable amount of non-specialised connective tissue is formed (cicatrisation).
In general, when the muscle weighs over 1.5 g, cicatrisation exceeds and overwhelms muscle regeneration. Optimum loading is that which on being applied generates mild discomfort during and after activity. Tissue regeneration does not occur in the absence of loading stimulus; low loading represented by resting conditions prevents the injury from getting worse, but does not improve the adaptation processes. High repeated loading, such as running more kilometres than usual, slows regeneration down and causes adaptations of the nervous system that can lead to more pain and to avoidance phenomena, as well as compensatory movement patterns(38,39,40,41).
A number of systems have been developed for classifying musculoskeletal injuries.
Traditionally, three degrees of rupture have been considered: mild, partial or complete. This system offers little information on the prognosis and required treatment, however.
Other classification systems have been developed in recent years, such as the Munich consensus or the classification of Chan et al.(35,36). The main problem with these classifications is the lack of objectiveness in the times of recovery or return to sports activities and treatment.
Lastly, the British Athletics Muscle Injury Classification proposes a new system based on the prognosis and therapeutic decision(35). The injuries are scored from 0-4 according to their magnetic resonance imaging characteristics, with the use of a suffix (a, b, c) according to the location involved: myofascial, musculoskeletal or intratendinous. This system was developed to classify ischiotibial muscle injuries, based on the literature in this field, but it may be extrapolatable to the rest of muscle injuries, in the same way as the Fútbol Club Barcelona Muscle Injury Guide: Prevention of and Return to Play from Muscle Injuries(36). However, we need greater diagnostic precision, a system affording more details regarding rehabilitation treatment, and a more defined time scale for application to settings as demanding as elite sports.
Treatment strategies
Determination of the most effective rehabilitation program for functional and muscle tissue recovery is crucial in order to minimise the risk of new injuries and to optimise athlete performance.
In the initial acute phase (3-5 days from the time of injury), treatment in the form of reduced activity, the application of ice, compression and limb elevation seeks to control the inflammatory phase. Early functional activation has been shown to increase vascularisation, improve muscle fibre regeneration, avoid fibrous scars, and more easily recover the viscoelastic and contractile characteristics of the muscle(38,39).
It has been shown that neuromuscular control exercises(40,41) and eccentric training(38) reduce the risk of ischiotibial tendon injury, and many authors recommend their inclusion as part of rehabilitation following injury due to acute exertion. It is believed that eccentric strengthening in particular increases distensibility of the series of muscles and allows longer operating periods, which can counter the effects of the scar tissue. It must be taken into account that, following the return to sports activity, the patient should be included in a progressive readaptation program to prevent and reduce the risk of new injuries(39).
Conclusions
The architecture and histology of tendon and muscle tissue are complex, and in combination with innervation and neurotransmission can allow us to understand the mechanobiology of these tissues. Their knowledge is the cornerstone on which to focus a correct and detailed diagnosis, as well as thorough, multidisciplinary and personalised treatment.
Figuras
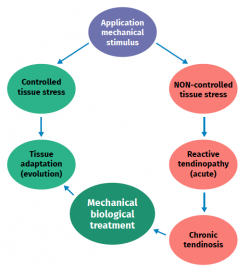
Figure 2. Schematic representation of the stimulation, stress, adaptation or lesion process in tendon physiology.
Figure 3. Schematic representation of tendon mechanical behaviour during mechanical stimulation. Adapted by F. Abat de Chicharro et al. Fisiología del ejercicio. Madrid: Ed. Médica Panamericana; 2006(29).
Figure 4. Example of multigym type isoinertial machine exercising to produce eccentric overload of the muscles of the lower extremities.
Tablas
Información del artículo
Cita bibliográfica
Autores
Ferrán Abat González
Traumatólogo deportivo, ReSport Clinic Barcelona.
Universidad Blanquerna, Ramon Llull. Barcelona
Servicio de Cirugía Ortopédica y Traumatología. Hospital de la Santa Creu i Sant Pau. Universitat Autònoma de Barcelona
Antonio Turmo Garuz
Centro de Alto Rendimiento de Sant Cugat. Universidad de Barcelona
Jocelio Campos Moraes
ReSport Clinic Barcelona. Universidad Blanquerna, Ramon Llull. Barcelona
Bruno Capurro Soler
ReSport Clinic Barcelona. Universidad Blanquerna, Ramón Llull. Barcelona
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved human or animal experimentation.
Data confidentiality. The authors declare that the protocols of their work centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology - a minireview of basic concepts and recent advancements. J Hand Ther. 2012 Apr-Jun;25(2):133-40.
-
2Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010 May;6(5):262-8.
-
3Banes AJ, Tsuzaki M, Yamamoto J, et al. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol. 1995 Jul-Aug;73(7-8):349-65.
-
4Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44(3-4):181-7.
-
5Screen HR, Shelton JC, Bader DL, Lee DA. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem Biophys Res Commun. 2005 Oct 21;336(2):424-9.
-
6Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007 Aug;88(4):217-26.
-
7Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010 May;28(5):639-43.
-
8Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010 Feb;28(2):198-203.
-
9Ackermann PW, Salo PT, Hart DA. Neuronal pathways in tendon healing. Front Biosci. 2009 Jun 1;14:5165-87.
-
10Ackermann PW. Neuronal regulation of tendon homeostasis. Int J Exp Pathol. 2013 Aug;94(4):271-86.
-
11Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173-209.
-
12Banes AJ, Tsuzaki M, Yamamoto J, et al. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol. 1995 Jul-Aug;73(7-8):349-65.
-
13Magra M, Hughes S, El Haj AJ, Maffulli N. VOCCs and TREK-1 ion channel expression in human tenocytes. Am J Physiol Cell Physiol. 2007 Mar;292(3):C1053-60.
-
14Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002 Mar;35(3):303-9.
-
15Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014 Oct;39:25-32.
-
16Jones E, Legerlotz K, Riley G. Mechanical regulation of integrins in human tenocytes in collagen and fibrin matrices. Bone Joint J. 2014; 96-B:161.
-
17Lavagnino M, Arnoczky SP, Gardner K. In situ deflection of tendon cell-cilia in response to tensile loading: an in vitro study. J Orthop Res. 2011 Jun;29(6):925-30.
-
18Docking SI, Daffy J, van Schie HT, Cook JL. Tendon structure changes after maximal exercise in the Thoroughbred horse: use of ultrasound tissue characterisation to detect in vivo tendon response. Vet J. 2012 Dec;194(3):338-42.
-
19Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech. 2012 Jan 3;45(1):59-65.
-
20Egerbacher M, Arnoczky SP, Caballero O, Lavagnino M, Gardner KL. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Relat Res. 2008 Jul;466(7):1562-8.
-
21Wang JH, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44(3-4):128-33.
-
22Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563-82.
-
23Abat F, Alfredson H, Cucchiarini M, et al. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part I: biology, biomechanics, anatomy and an exercise-based approach. J Exp Orthop. 2017 Dec;4(1):18.
-
24Roig M, O'Brien K, Kirk G, et al. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009 Aug;43(8):556-68.
-
25Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9(2):493-5.
-
26Järvinen TA, Järvinen TL, Kääriäinen M, et al. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol. 2007 Apr;21(2):317-31.
-
27Best TM, Hunter KD. Muscle injury and repair. Phys Med Rehabil Clin N Am. 2000 May;11(2):251-66.
-
28Casey A, Greenhaff PL. Does Dietary Creatine Supplementation Play a Role in Skeletal Muscle Metabolism and Performance? Am J Clin Nutr. 2000 Aug;72(2 Suppl):607S-17S.
-
29Chicharro J, Fernández A. Fisiología del ejercicio. Madrid: Ed. Médica Panamericana; 2006.
-
30Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol (1985). 2005 May;98(5):1900-8.
-
31Huijing PA, Jaspers RT. Adaptation of muscle size and myofascial force transmission: a review and some new experimental results. Scand J Med Sci Sports. 2005 Dec;15(6):349-80.
-
32Ekstrand J, Hägglund M, Waldén M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011 Jun;39(6):1226-32.
-
33Williams S, Trewartha G, Kemp S, Stokes K. A meta-analysis of injuries in senior men's professional Rugby Union. Sports Med. 2013 Oct;43(10):1043-55.
-
34Feddermann-Demont N, Junge A, Edouard P, Branco P, Alonso JM. Injuries in 13 international Athletics championships between 2007-2012. Br J Sports Med. 2014 Apr;48(7):513-22.
-
35Pollock N, James SL, Lee JC, Chakraverty R. British athletics muscle injury classification: a new grading system. Br J Sports Med. 2014 Sep;48(18):1347-51.
-
36Valle X, Alentorn-Geli E, Tol JL, et al. Muscle Injuries in Sports: A New Evidence-Informed and Expert Consensus-Based Classification with Clinical Application. Sports Med. 2017 Jul;47(7):1241-53.
-
37Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990 Aug;22(4):429-35.
-
38Petersen J, Thorborg K, Nielsen MB, Budtz-Jørgensen E, Hölmich P. Preventive effect of eccentric training on acute hamstring injuries in men's soccer: a cluster-randomized controlled trial. Am J Sports Med. 2011 Nov;39(11):2296-303.
-
39Silder A, Sherry MA, Sanfilippo J, Tuite MJ, Hetzel SJ, Heiderscheit BC. Clinical and morphological changes following 2 rehabilitation programs for acute hamstring strain injuries: a randomized clinical trial. J Orthop Sports Phys Ther. 2013 May;43(5):284-99.
-
40Cameron ML, Adams RD, Maher CG, Misson D. Effect of the HamSprint Drills training programme on lower limb neuromuscular control in Australian football players. J Sci Med Sport. 2009 Jan;12(1):24-30.
-
41Kraemer R, Knobloch K. A soccer-specific balance training program for hamstring muscle and patellar and achilles tendon injuries: an intervention study in premier league female soccer. Am J Sports Med. 2009 Jul;37(7):1384-93.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Tendon injury: from eternally forgotten to trendy disease
- Physiology and mechanobiology of tendon and muscle tissue
- Patellar tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Pathophysiology, diagnosis and treatment of Achilles tendinopathy
- Supraspinatus tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Results of arthroscopic gluteus medius tendon repair in patients with trochanteric pain syndrome. Case series
- Update on the diagnosis and treatment of quadriceps muscle injuries
- Management of triceps surae muscle injuries in young adult and middle-aged athletes: a narrative literature review
- Insertional tendinopathy of the Achilles tendon. Treatment from start to finish
- Inverted cyclops
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.