Insertional tendinopathy of the Achilles tendon. Treatment from start to finish
Tendinopatía insercional del tendón de Aquiles. Tratamiento de principio a fin
Resumen:
La tendinopatía insercional del tendón calcáneo es un trastorno frecuente, asociado con el aumento de la edad y con los microtraumatismos repetidos por el sobreuso del tendón en pacientes con factores predisponentes. La presencia de lesiones en las bursas, la formación de calcificaciones y las lesiones en la estructura del propio tendón son características.
La mayoría de los estudios disponibles sugieren que los pacientes con tendinopatía insercional deben tratarse de inicio con medidas conservadoras, durante un periodo de 3 a 6 meses, entre las que se incluyen un programa de ejercicios dirigidos, la terapia con ondas de choque extracorpóreas de alta energía y el tratamiento de fisioterapia.
Los tratamientos quirúrgicos incluyen actuaciones de desbridamiento y resección de bursas inflamadas, de tejido sinovial hipertrófico, de calcificaciones insercionales, del tejido degenerativo intratendinoso, la resección de prominencias de la tuberosidad posterior y la reinserción del tendón de Aquiles. Las complicaciones postoperatorias no son infrecuentes y los problemas de cicatrización de las heridas son una de las complicaciones más comunes. El tratamiento quirúrgico de la tendinopatía insercional del tendón de Aquiles resuelve con frecuencia el dolor y mejora la funcionalidad. La rehabilitación completa puede alcanzarse hasta un año después de la intervención.
Abstract:
Insertional tendinopathy of the calcaneal tendon is a common disorder associated with advancing age and recurrent microtraumatisms due to overuse of the tendon in patients with predisposing factors. The presence of lesions in the bursae, the formation of calcifications, and damage to the tendon structure itself are common findings.
Most studies in the literature suggest that patients with insertional tendinopathy should initially be managed on a conservative basis during a period of 3-6 months, including guided exercise programs, high-energy extracorporeal shock wave therapy, and physiotherapy.
The surgical treatment options in turn include debridement and resection of inflamed bursae, hypertrophic synovial tissue, insertional calcifications and intratendinous degenerative tissue, the resection of prominences of the posterior tuberosity, and reinsertion of the Achilles tendon. Postoperative complications are not infrequent, with wound healing problems being one of the most frequent complications. Surgical treatment of insertional tendinopathy of the Achilles tendon often resolves the pain and improves function. Complete rehabilitation may be reached up to one year after the operation.
Introduction
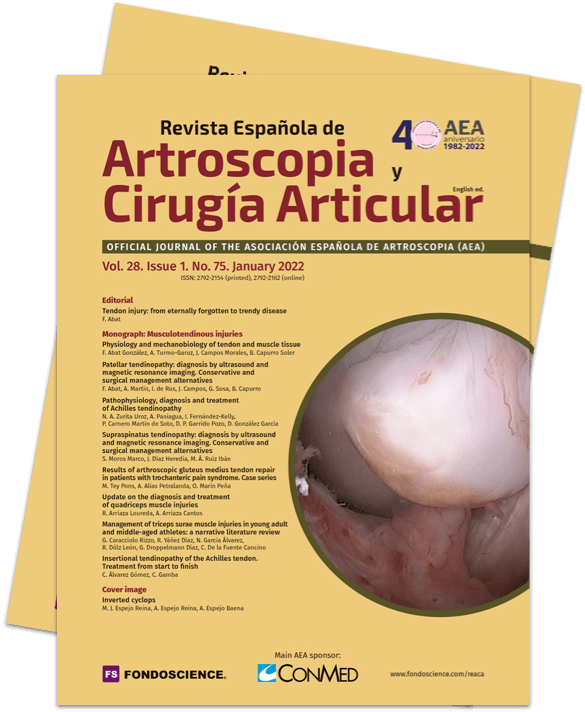
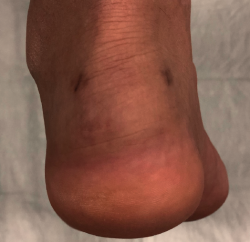
Approximately 6% of the general population will experience some pain episode of the Achilles tendon during life(1). Of these patients, approximately one-third will present tendon insertion damage(2,3). Patients with insertional tendinopathy of the Achilles tendon (ITA) typically experience pain and functional limitation, aggravated by physical activity and following resting periods. They may present thickening of the insertional portion of the tendon, most often at the lateral margin (Figure 1).
Different anatomical structures intervene in insertional disorders, and the clinical manifestations are diverse and subtle.
The present chapter describes definitions and classifications, and seeks to summarize the treatment options.
Terminological consensus
The terminology used over the years has been heterogeneous, due to the different clinical presentations and conditions that characterise the structures adjacent to the insertion of the tendon, and this sometimes gives rise to confusion.
The terms "Haglund's syndrome", "Haglund's deformity" or "Haglund's disease" have been used in an imprecise manner for some time(4,5,6).
In order to establish more uniform definitions and know what disease condition is being referred to in each case, Van Dijk et al.(7,8) proposed a terminological consensus based on the anatomical location, symptoms, clinical findings and pathological changes of each condition:
- Insertional tendinopathy: tissue damage to the tendon itself, at its calcaneal insertion, with the possible presence of bone enthesophytes and calcifications in the tendon. The symptoms comprise pain in response to palpation, an increase in volume, and functional limitation with mechanical pain. The histopathological findings consist of ossification of the fibrocartilage of the enthesis and, occasionally, partial rupture of the tendon at the tendon-bone interface.
- Retrocalcaneal bursitis: inflammation of the bursa located in the recess between the anteroinferior portion of the Achilles tendon and the posterosuperior surface of the calcaneus (retrocalcaneal recess). This condition results in visible and painful inflammation of the soft tissues medial and/or lateral to the tendon at the height of the posterosuperior region of the calcaneus. The plain radiographs often reveal a prominence of the posterosuperior tuberosity of the calcaneus. The histopathological findings comprise degeneration of the walls of the bursa, with synovial hypertrophy and presence of fluid.
Retrocalcaneal bursitis is often seen in combination with insertional tendinopathy. - Superficial bursitis of the calcaneus: inflammation of the bursa located between the skin and the junction of the tendon with the posterior tuberosity of the calcaneus. There are no intratendinous alterations. The disorder may be selectively posterolateral or posteromedial.
As far as possible, it is preferable to not use the terms employed in the past (such as "Haglund's syndrome" or pump bump), in order to avoid the confusion found in the scientific community regarding the precise and consensus-based definition, diagnosis and treatment of these disorders.
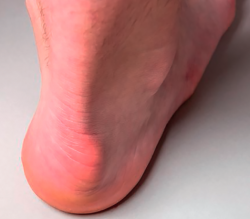
Knowing the structures involved, the topographic anatomy is very useful in this location (Figure 2).
Pathomechanics
Multiple factors are involved in the origin of enthesopathic injuries of the calcaneal tendon, including intrinsic factors such as dysmetria or misalignment of the lower extremities, mobility defects of the ankle, or morphological variants and deformities of the foot, which play an important role(9,10,11). The extrinsic factors in turn involve mechanical overload or excessive tendon use(12). At tissue level, both the magnitude of the force and the tension in the tendon associated to physical activity contribute to generate stress in the insertion of the tendon. Running generates loads of 4-6 times of the body weight upon the Achilles tendon. Other factors such as advanced age, diabetes or rheumatic diseases also seem to exert an effect(13).
Intratendinous degenerative changes in the insertion are characteristic of ITA. Such changes must be discarded in patients with consistent clinical manifestations. Degeneration of the tendon is characterised by the loss of collagen parallel structure, loss of fibre integrity, fat infiltration and capillary proliferation(14,15). Clinically, thickening of the tendon is noted.
The development of osteophytes is a response to cumulative mechanical loading with age and/or activity(16,17,18).
Superficial calcaneal bursitis is often associated to the wearing of shoes with a rigid posterior element. This condition represents an adventitious bursa acquired after birth and which develops in response to friction.
Shortening of the gastrocsoleus complex may be present in up to 20% of all cases of insertional tendinopathy, though its presence is much more important in non-insertional tendinopathies.
Diagnosis
Clinical presentation
The diagnosis is essentially established from the case history and physical examination. Anatomical inspection of the patient is required to assess the clinical signs, with an analysis of the relevant history of disorders of the locomotor apparatus along the entire lower extremity. Evaluation is required of the gait pattern, alterations of the gastrocnemius-Achilles-plantar complex, plantar flexion weakness and misalignments of the ankle and foot. The patient usually experiences pain in response to palpation over the distal 2 cm of the tendon, with worsening of the pain after physical activity and stiffness with pain following resting periods(19). The diagnosis is clinical, but should be supported by radiological tests.
Imaging tests
Plain radiography
The dorsoplantar and sagittal views of both feet under loading conditions are indicated, together with the posteroanterior view of the ankles under loading conditions.
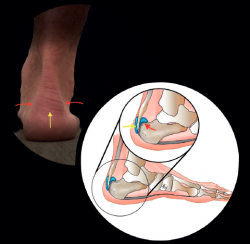
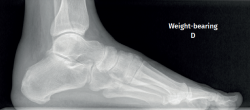
Plain radiography under loading conditions is the essential initial diagnostic tool(20). It allows us to assess alignments and dysmetria, to measure angles, to determine the morphology of the calcaneus and its posterior tuberosity, to characterise bone exostosis and increments in thickness of the retrocalcaneal soft tissues, and to plan surgical procedures (Figure 3).
Ultrasound
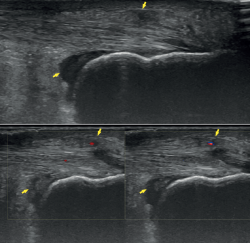
Ultrasound allows us to identify inflammatory signs in both bursae, tendon lesions and bone exostosis. Tendon degeneration is characterised by poorer echogenicity (a lower mean value on the scale of greys), due to the lesser organisation and altered composition of the tendon microstructure(21). Doppler ultrasound in turn allows us to assess neovascularisation zones (Figure 4). Ultrasound offers dynamic exploration and is operator-dependent.
Magnetic resonance imaging
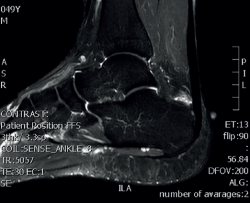
Magnetic resonance imaging (MRI) allows us to evaluate the presence of intratendinous injuries, fluid collections, the appearance of the bursae, bone edemas in the posterior tuberosity, and to establish the differential diagnosis with other disease conditions(22) (Figure 5). If the plain radiographs and ultrasound findings are conclusive, MRI is not essential. In the absence of an experienced ultrasound operator, or if there are doubts after ultrasound regarding the presence of disease in the tendon and bursa, MRI is very useful.
Computed tomography
Computed tomography (CT) allows more concise evaluation of intratendinous calcifications or enthesophytes (whether fragmented or not), and is also useful for establishing the differential diagnosis with other disorders. It is not necessary to request CT evaluation on a routine basis.
Signs of tendinopathy in the imaging techniques are not necessarily indicative of the presence of clinically symptomatic disease and vice versa, as reported in different studies(23,24). The percentage of asymptomatic tendons with signs of Achilles tendinopathy in the imaging studies ranges from 0-35%, while the percentage of symptomatic tendons without signs of tendinopathy ranges from 0-19%(23,25,26,27,28,29).
Baropodometric study
A good biomechanical study will allow us to analyse anomalies of the gait or running pattern, to characterise valgus misalignments, hyperpronation of the foot in some cases, or alterations inherent to cavus foot. There may be functional alterations of the capacity for dorsiflexion and plantar flexion of the ankle, with a lesser range and decrease in plantar flexor power.
Retraction of the gastrocnemius-Achilles complex is evident at the end of the second rocker of gait and can be diagnosed with the Silfverskiöld test.
Conservative management
Initial management for injuries of this kind requires a multidisciplinary approach with the required cohesion among the orthopaedic surgeon, the physiotherapy team and the podiatrist to identify the different intrinsic and extrinsic factors that give rise to problems of this type.
Following a detailed evaluation of the clinical data obtained from the diagnostic process, and in accordance with the consensus document published by the European Society for Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA) Science Basic Committee, the authors recommend conservative management during 3-6 months before considering surgical treatment(30).
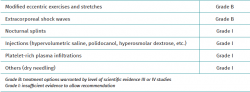
Most nonsurgical treatments for Achilles enthesopathy lack the required supporting evidence to recommend their use, though guided physiotherapy and extracorporeal shockwaves (ESWT) are exceptions (Table 1)(31,32).
Guided exercise
Treatment in the form of exercise and stretching targeted to the insertional region of the tendon has the highest level of recommendation for reducing pain in this location (grade of recommendation B)(1). The effects of exercise can be improved by using a broad range of other treatments, including soft tissue therapy, nutritional supplements, iontophoresis, the modification of physical activity, drug treatments and raising of the heel with plantar support (grade of recommendation I)(33,34,35).
There is no consensus on the ideal strategy and parameters of exercise (type, frequency or combination with additional treatments). It has been shown that therapy in the form of eccentric exercises, which proves useful in non-insertional tendinopathy, causes pain in many patients with enthesopathy. It is therefore contraindicated if pain is produced. In this regard, it has been suggested that a reduced ankle dorsiflexion range, without exceeding the neutral position as a modification of classical eccentric exercise, might be of benefit for patients of this kind(30,36). In addition to modified eccentric exercise limited in its range for these patients, it is advisable to alternate with concentric and isometric work, and to apply inertial slow resistance elements(37,38).
Local anaesthetic injections may be regarded particularly as a means to facilitate patient participation in an exercise program (grade of recommendation I)(1).
Shockwave therapy
When exercise does not prove successful, ESWT seems to be the best next nonsurgical treatment option for reducing the pain (grade of recommendation B)(1,39,40,41).
Some studies have reported better outcomes with ESWT in terms of pain reduction than with a traditional eccentric exercise program after 6 and 18 months of follow-up(42). The risk of ESWT is intense pain during the treatment, which may cause the patient to abandon such therapy. The use of a local anaesthesia may help patients to better tolerate the treatment(43).
Shockwave therapy in combination with exercise offers better results, including pain relief, functional improvement and a return to usual activities. Patients with a higher level of sports activity would have better therapeutic responses than less active patients(44).
Footwear adjustment
There is some biomechanical evidence which suggests that raising of the heel can reduce the tensile and compression forces in the tendon during daily activities that require dorsal flexion of the ankle(45,46). However, excessive shortening of the triceps surae is to be avoided. Patients with alterations of the Achilles tendon often present concomitant misalignment of the hindfoot. Foot orthoses may be useful in such patients. In the case of athletes, modification of the rear part of their footwear may sometimes prove effective.
Infiltrations
In current clinical practice, corticosteroid injections are not indicated for the treatment of tendinopathy in any location. In particular, the concern referred to the Achilles tendon is that they may contribute to greater tendon degeneration and possible rupture(47). In cases of isolated retrocalcaneal bursitis, high satisfaction rates have been reported with selective infiltrations of the bursa, though the risk of iatrogenic effects upon the tendon are evident, due to its proximity(48). Other possible types of injections include those targeted to neovascularisation, such as the sclerosing agent polidocanol or hypervolumetric injections of saline solution and hyperosmolar dextrose(49). Although a number of small trials have evaluated these agents, most of them do not specifically focus on the insertional zone of the Achilles tendon and do not contribute evidence for their use in routine clinical practice(48).
Platelet-rich plasma (PRP) infiltrations currently do not have sufficient evidence for use in this particular location(50,51,52,53,54,55). There is still a lack of standardisation referred to the obtainment and method of application of PRP. To date, most studies using PRP for the Achilles tendon have been carried out in patients with non-insertional tendinopathy(56). Randomised double-blind studies are needed, with a greater level of evidence, before a level of recommendation can be established for treatment with PRP (current grade of recommendation I). Studies in basic science may prove crucial in providing biological justification for the safe clinical use of PRP. Its use is therefore subject to controversy.
Dry needling
Dry needling is another nonsurgical treatment option for patients with insertional tendinopathy. Some studies have reported pain relief and improved functional scores following dry needling treatment, which is moreover well tolerated(57).
Surgical treatment
Surgical treatment differs depending on the case. Individualisation of the management of each patient is therefore very important in this anatomical region. According to the three different clinical presentations summarised in the terminological consensus section, treatment should focus on resolving problems of the tendon and of the bursae, with action upon deformities or bone prominences, or interventions at different levels.
Superficial calcaneal bursitis
In patients with inflammatory conditions of the superficial bursa of the calcaneus (retroachilleal zone), conservative management and modification of footwear are the cornerstones of treatment, and the healing rate without the need for surgery is greater than in the other conditions that are present in the insertion.
The patient typically reports clearly identifiable pain in the most superficial plane of the insertion. Inspection reveals thickening of the preachilleal tissues and conflict on wearing shoes with a hard counter or buttress. In cases of isolated preachilleal bursitis that fails to improve with conservative management, open exeresis is the treatment of choice(58).
Retrocalcaneal bursitis
In the case of patients diagnosed with retrocalcaneal bursitis and presenting spatial conflicts with the posterior tuberosity of the calcaneus, in the absence of symptomatic tendon disease, calcaneoplasty is the surgical technique usually employed.
Calcaneoplasty offers access to the retrocalcaneal space, which allows us to resect the inflamed retrocalcaneal bursa and the synovitis tissue, as well as generate the space needed for resecting the posterosuperior prominence of the calcaneus.
Open calcaneoplasty
The standardised technique makes use of an open longitudinal lateral approach measuring about 4 cm in size. By dissecting the corresponding anatomical planes, we reach the fascia and perform an incision longitudinally to gain access to the posterior chamber, where the inflamed bursa and the reactive synovial tissue are removed. Then, the prominence of the posterior tuberosity is resected by means of an osteotomy, to avoid compression with the ventral portion of the Achilles tendon(59).
Endoscopic calcaneoplasty
In 2001, Van Dijk et al. described the standardised endoscopic technique for this type of procedure(60).
The patient is placed in prone decubitus. The ischemia cuff is placed on the thigh. It is advisable to use a supporting device beneath the distal tibia to ensure a knee flexion of about 15°. The foot and ankle should be left hanging from the end of the operating table to allow free range of motion. The skin reference points are the distal tip of the lateral malleolus, the Achilles tendon, and the posterior tuberosity of the calcaneus. The classical endoscopic portals of the hindfoot (posteromedial and posterolateral) are modified and are located slightly distal and anteriorised, with the purpose of securing improved access and visualisation of the tendon insertion zone (Figure 6).
reacae.29175.fs2108027en-figure7.png

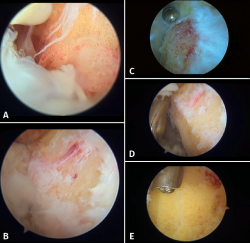
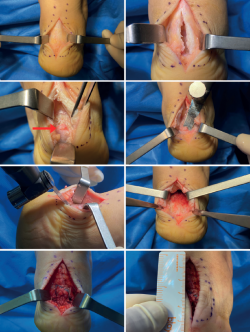
Figure 7. Endoscopic images. A: bursitis and synovitis; B: initial debridement and creation of the working field. Visual access to the posterior tuberosity; C: debridement and calcaneoplasty with synoviotome; D: access to the most distal portion, protection of the tendon fibres with the blunt part and calcaneoplasty; E: use of a protected powered drill for bone levelling.
We first establish the posterolateral visioning portal and introduce the instruments through the posteromedial portal. The standard instrument used is the 4.5 mm at 30° arthroscope. The endoscopic procedure is carried out with an irrigation pump. Through the posterolateral portal we first introduce a mosquito forceps and then the arthroscope obturator. It is useful to perform back and forth movements within Kager's space and release medially, to secure better space and a good working field. The optics are placed looking to the medial margin, and a needle is used to calibrate the ideal position of the medial portal. An endoscopic technique is used to debride the soft tissues of the posterior chamber in order to create a working space around the calcaneus and the tendon insertion zone. We usually identify hypertrophic synovial tissue and a bursitis area in the retrocalcaneal space (Figure 7A and 7B). Debridement is performed using a synoviotome in contact with the upper zone of the calcaneus. While the tissue is resected and the operation progresses, we must protect the tendon fibres with the blunt part of the working instruments (Figure 7D).
Once the soft tissues have been resected, we must inspect the ventral zone of the tendon to discard tissue lesions requiring specific treatment.
Then, where applicable, and combining the drill and synoviotome, we resect the bony prominence of the tuberosity (Figure 7C and 7E).
We should focus on the most lateral and medial margins, as these are the most common zones of conflict. In this regard, it is useful to exchange the portals to ensure access to both sides and allow sufficient resection to be performed. During or at the end of the process, we can use radiological control (sagittal view) for assistance to check the resection plane achieved. Endoscopic vision should evidence decompressive relief upon the tendon with maximum dorsal flexion and mobilisation manoeuvres. The insertion of the tendon marks the limit of resection.
One of the difficulties of the endoscopic technique is identification of the amount of tissue and exostosis requiring resection. If debridement is extensive, includes the resection of calcifications, and threatens the integrity of the insertion of the tendon, Vega et al.(61) propose an endoscopic technique for tendon augmentation using anchors and sutures. The incidence of wound complications in the endoscopic procedure is extremely low.
In our experience, the main indication of endoscopic calcaneoplasty would refer to cases with a posterior bony prominence compressing the anterior insertional area of the tendon, with retrocalcaneal bursitis and reactive synovial tissue.
These are usually patients with pain in response to lateral and medial palpation of the Achilles tendon in the region in contact with the posterosuperior tuberosity of the calcaneus.
However, in cases presenting with pain and degenerative signs of the tendon itself, or with the presence of symptomatic enthesophytes, the percentage of good outcomes of calcaneoplasty decreases significantly, and other surgical options are preferred.
General postoperative recommendations
- First 48 hours: resting with the foot raised. Suropedic compression bandage.
- Third postoperative day: wound cure and switch to a new elastic compression bandage.
- Weeks 1 to 6: range of motion exercises, stretching, massotherapy and partial weight-bearing with boot (up to 50% of total body weight) in the first two weeks.
- Week 3: suture removal.
- From week 3: progressive full weight-bearing. Alternate boot with sports footwear according to tolerance. Begin work with stationary bicycle at the start of the week 4 after surgery.
- Weeks 7 to 12: walking without restrictions or aids. Moderate intensity bicycle work. Start exercises for balance and coordination, triceps surae isometric, modified eccentric and concentric exercises, with restriction of dorsal flexion of the ankle.
- From week 12: alternate intervals of short runs and walking.
- From month 4: sports activities with maximum mechanical demands, according to tolerance(59).
Attributing endoscopic procedures with early recovery may lead to error or false expectations. In the context of insertional disease, in those procedures requiring bone surgery of the calcaneus combined with soft tissue debridement, the full resolution of patient discomfort may take up to one year after surgery. Likewise, reaching maximum competition level for a professional may take from several months to one year.
Insertional tendinopathy
In patients with symptomatic conditions of the insertion of the tendon, in the presence of an intratendinous enthesophyte or a fragmented free osteophyte, we advocate open surgery using a central approach. Other approaches have also been described for accessing the insertional zone(62). They allow access to the affected region of the tendon, the possibility to resect osteophytes, treat the tendon, perform osteotomy of the posterosuperior tuberosity and effectively re-anchor the portion of tendon subjected to surgical treatment.
reacae.29175.fs2108027en-figure8.png

Figure 8. Longitudinal incision measuring about 6 cm and central opening of the tendon, preserving lateral insertions. Exposure of the distal portion of the tendon fibres, visualisation of calcifications (red arrow), bursae, synovial hypertrophy and posterior tuberosity of the calcaneus. Debridement of metaplastic tendon tissue and bursa, removal of calcifications, levelling and ostectomy of the posterior tuberosity. Reinsertion.
A longitudinal incision measuring about 6-8 cm is made centred on the midline. In some cases we may curve the distal segment of the incision if there are alterations of the lateral or medial side of the tendon insertion(63). The subcutaneous layer is dissected, taking special care not to damage the neurovascular structures. We must try to maintain continuous tissue thickness to the paratenon in order to avoid surgical wound healing complications. A longitudinal incision is made in the most distal segment of the paratenon, performing dissection with respect to the tendon. In the case of thickening and preachilleal bursitis, debridement is indicated. Longitudinal division is made through a midline incision, and we check the presence of tendinous degenerative changes, intratendinous calcifications, retrocalcaneal synovitis and bursitis, or degenerative lesions in the enthesis (Figure 8). Debridement and levelling of the lesions are performed. Incomplete debridement and the persistence of enthesophytes has been associated to an increased risk of persistent symptoms and lower satisfaction rates after surgery(64). The posterior tuberosity is exposed, and osteotomy is performed using an osteotome or oscillating saw to fully eliminate the spatial conflict with the tendon and check its decompression clinically and, if necessary, radiologically.
We must check complete resection of the medial or lateral calcifications in the segments where insertion of the tendon fibres in the calcaneus is preserved. It is important to preserve part of the insertion to precisely determine the re-anchoring zone.
In cases characterised by severe degeneration of the Achilles tendon, with scant repair capacity, transfer of the flexor hallucis longus (FHL) is required.
Finally, reinsertion of the Achilles tendon is carried out. Many techniques have been described. Our preferred approach is knot-free reconstruction with four anchors and a double parallel and crossed row, using high-resistance tapes, since they have been shown to be mechanically superior to other options(65,66,67,68,69,70,71,72). The foot is kept in plantar flexion while adequate tension is achieved. Posteriorly, we close the longitudinal opening of the tendon and paratenon individually (Figure 9).
Following wound closure, immobilisation is carried out in plantar flexion of about 15-20°.
General postoperative recommendations
- First 48 hours: resting with the foot raised. Posterior splint.
- Third postoperative day: wound cure and maintain splint.
- Between weeks 2 and 3: remove splint and sutures. Conversion to weight-bearing boot orthosis.
- Weeks 3 to 5: range of motion exercises, stretching, local physical therapy to reduce symptoms and partial weight-bearing with boot according to tolerance.
- From week 5: progressive full weight-bearing and gradual conversion to sports footwear. Start work with stationary bicycle.
- Weeks 7 to 12: moderate intensity bicycle work. Start isometric, balance and coordination exercises.
- From week 12: supervised modified eccentric and concentric exercises only to the horizontal plane of the ankle. Start short runs. High intensity bicycle work.
- Months 4 to 6: progressive return to routine sports activities according to adherence to functional rehabilitation treatment.
An immunohistochemical study of 10 biopsies from patients with ITA evidenced a high degree of innervation of both bursae (subcutaneous and retrocalcaneal), of the resected posterior tuberosity, and of the degenerative tendon fibres(73).
The insertional calcifications recurrence rate has not been well studied. Nunley et al.(74) conducted a retrospective review of 29 surgical procedure in 27 patients (mean duration of follow-up 4 years; range 2.9-8.1 years). At last follow-up, the lateral radiographs showed recurrent calcifications in 11 feet, though all the patients were pain-free despite the radiological findings.
Incomplete resection and debridement of the pathological tendon zones, of the calcifications and of the posterior tuberosity may lead to persistence of the symptoms after surgery.
The surgical treatment of ITA is associated to a postoperative complications rate that needs to be taken into account. Wound healing problems are the most common complications, with an incidence of up to 30% according to some series. These problems are unusual with the endoscopic technique, however(64).
Transfer of the FHL as augmentation measure can be made endoscopically or on an open basis, depending on the technique chosen for the procedure, in those cases with advanced degeneration of the tendon. The evidence supporting the routine use of this procedure in patients with ITA is presently insufficient(75).
There is no evidence on the effectiveness of the surgical elongation of the gastrocnemius complex in patients with ITA. Improved research studies are needed in this field(1).
Most patients subjected to surgical treatment report pain relief and functional improvement after the operation. However, the percentage of patients that become totally pain-free does not reach 100%, according to the published reviews of case series(64). The postoperative rehabilitation time until the return to sports activities and substantial pain relief may be quite long.
Osteotomy of the calcaneus with dorsal subtraction wedge (modified Zadek procedure)
In cases with a calcaneal inclination angle of over 20° and a calcaneal X/Y ratio (as proposed by Tourne et al.) of under 2.5, good outcomes have been reported with this surgical technique, though it is not as widely used in routine practice as the other techniques(76,77). Shortening of the calcaneus would minimise posterior leverage of the gastrocnemius-Achilles complex and, together with displacement of the tuberosity, would alleviate the tension produced in the tendon insertion.
Conclusions
There is strong consensus in the available literature regarding the need for initial conservative management of patients with ITA. In cases unresponsive to exhaustive conservative management during a period of 3 to 6 months, individualised surgery adjusted to each case affords good outcomes. In the case of patients with retrocalcaneal bursitis, signs of compression in the zone of the posterior tuberosity and without symptoms in the tendon or enthesophytes, endoscopic or open calcaneoplasty is the technique of choice. In patients with intratendinous degenerative disease, in combination with the finding of calcifications and bursitis, open surgery at tendon insertion level is the treatment of choice.
Figuras
Figure 1. Insertional tendinopathy of the Achilles tendon. Thickening of the soft tissues, prominence on the external side.
Figure 2. Topographic correlation. Distal central pain: tendinopathy, preachilleal bursitis, enthesophytes (yellow arrows). Lateral and medial pain: retrocalcaneal bursitis, conflict with the posterior calcaneal tuberosity (red arrows). Illustration authorship: Dr. Álvaro Fernández.
Figure 3. Plain radiography, Sagittal view with weight-bearing. Moderate calcifications in the insertion of the Achilles tendon with intratendinous enthesophyte. Morphological alterations of the posterior tuberosity of the calcaneus.
Figure 4. Ultrasound exploration. Retrocalcaneal bursitis and signs of tendinopathy (yellow arrows). Doppler ultrasound allows the identification of tendon hypervascularisation areas.
Figure 5. Magnetic resonance imaging. Retrocalcaneal bursitis. Fluid in the retrocalcaneal recess without signs of degenerative tendinopathy.
Figure 7. Endoscopic images. A: bursitis and synovitis; B: initial debridement and creation of the working field. Visual access to the posterior tuberosity; C: debridement and calcaneoplasty with synoviotome; D: access to the most distal portion, protection of the tendon fibres with the blunt part and calcaneoplasty; E: use of a protected powered drill for bone levelling.
Figure 8. Longitudinal incision measuring about 6 cm and central opening of the tendon, preserving lateral insertions. Exposure of the distal portion of the tendon fibres, visualisation of calcifications (red arrow), bursae, synovial hypertrophy and posterior tuberosity of the calcaneus. Debridement of metaplastic tendon tissue and bursa, removal of calcifications, levelling and ostectomy of the posterior tuberosity. Reinsertion.
Figure 9. Postoperative radiological control showing adequate resection of the posterior tuberosity and correct positioning of the anchors (biocomposite).
Tablas
Información del artículo
Cita bibliográfica
Autores
Carlos Álvarez Gómez
Servicio de Cirugía Ortopédica y Traumatología. Hospital Universitario Marqués de Valdecilla. Santander
Cirugía Ortopédica y Traumatología. Hospital de la Santa Creu i Sant Pau. Universitat Autònoma de Barcelona
Universitat Autònoma de Barcelona. Hospital Sanitas CIMA. Barcelona
Carlo Gamba
Cirugía Ortopédica y Traumatología. Parc de Salut Mar. Universitat Pompeu Fabra (UPF). Barcelona
Hospital del Mar. Barcelona
Editor asociado de REACA
Servicio de Cirugía Ortopédica y Traumatología. Unidad de Pie y Tobillo. Hospital del Mar. Barcelona
Clínica Corachan. Barcelona
Universitat Pompeu Fabra (UPF). Barcelona
Cirugía Ortopédica y Traumatología. Hospital de la Santa Creu i Sant Pau. Universitat Autònoma de Barcelona
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that the procedures carried out abided with the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed, and that all the patients included in the study have received sufficient information and have given written informed consent to participation in the study.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Chimenti RL, Cychosz CC, Hall MM, Phisitkul P. Current Concepts Review Update: Insertional Achilles Tendinopathy. Foot Ankle Int. 2017 Oct;38(10):1160-9.
-
2Kang S, Thodarson DB, Charlton TP. Insertional achilles tendinitis and Haglund’s deformity. Foot Ankle Int. 2012;33(6):487-91.
-
3Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (entheses) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471-90.
-
4Sella EJ, Caminear DS, McLarney EA. Haglund's syndrome. J Foot Ankle Surg. 1998 Mar-Apr;37(2):110-4; discussion 173.
-
5Ruch JA. Haglund's disease. J Am Podiatry Assoc. 1974 Dec;64(12):1000-3.
-
6Frey C. Surgical advancements: arthroscopic alternatives to open procedures: great toe, subtalar joint, Haglund's deformity, and tendoscopy. Foot Ankle Clin. 2009 Jun;14(2):313-39.
-
7Van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Mafulli N. Terminology for Achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):835-41.
-
8Opdam KTM, Zwiers R, Wiegerinck JI, van Dijk CN; Ankleplatform Study Collaborative–Science of Variation Group. Increasing consensus on terminology of Achilles tendon-related disorders. Knee Surg Sports Traumatol Arthrosc. 2021 Aug;29(8):2528-34.
-
9Chimenti RL, Flemister AS, Tome J, et al. Altered tendon characteristics and mechanical properties associated with insertional Achilles tendinopathy. J Orthop Sports Phys Ther. 2014;44(9):680-9.
-
10Den Hartog BD. Insertional Achilles tendinosis: pathogenesis and treatment. Foot Ankle Clin. 2009;14(4):639-50.
-
11Shibuya N, Thorud JC, Agarwal MR, Jupiter DC. Is calcaneal inclination higher in patients with insertional Achilles tendinosis? A case-controlled, cross-sectional study. J Foot Ankle Surg. 2012;51(6):757-61.
-
12Chimenti RL, Flemister AS, Tome J, McMahon JM, Houck JR. Patients with insertional Achilles tendinopathy exhibit differences in ankle biomechanics as opposed to strength and range of motion. J Orthop Sports Phys Ther. 2016;46(12):1051-60.
-
13D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, et al. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48(2):523-33.
-
14Klauser AS, Miyamoto H, Tamegger M, et al. Achilles tendon assessed with sonoelastography: histologic agreement. Radiology. 2013;267(3):837-42.
-
15Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand. 1997;68(2):170-5.
-
16Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum. 2000;43(3):576-83.
-
17De Jonge S, Tol JL, Weir A, et al. The tendon structure returns to asymptomatic values in nonoperatively treated Achilles tendinopathy but is not associated with symptoms: a prospective study. Am J Sports Med. 2015;43(12):2950-8.
-
18Franz JR, Slane LC, Rasske K, Thelen DG. Non-uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture. 2015;41(1):192-7.
-
19DeOrio MJ, Easley ME. Surgical strategies: insertional Achilles tendinopathy. Foot Ankle Int. 2008;29(5):542-50.
-
20Fowler A, Philip JF. Abnormality of the calcaneus as a cause of painful heel its diagnosis and operative treatment. Br J Surg. 1945;32(128):494-8.
-
21Khan KM, Forster BB, Robinson J, et al. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med. 2003;37(2):149-53.
-
22Syha R, Springer F, Wurslin C, et al. Tendinopathy of the Achilles tendon: volume assessed by automated contour detection in submillimeter isotropic 3-dimensional magnetic resonance imaging data sets recorded at a field strength of 3 T. J Comput Assist Tomogr. 2015;39(2):250-6.
-
23Bakkegaard M, Johannsen FE, Hojgaard B, Langberg H. Ultrasonography as a prognostic and objective parameter in Achilles tendinopathy: a prospective observational study. Eur J Radiol. 2015;84(3):458-62.
-
24Astrom M, Westlin N. Blood flow in the human Achilles tendon assessed by laser Doppler flowmetry. J Orthop Res. 1994;12(2):246-52.
-
25De Zordo T, Chhem R, Smekal V, et al. Real-time sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010;31(4):394-400.
-
26Fischer MA, Pfirrmann CW, Espinosa N, Raptis DA, Buck FM. Dixon-based MRI for assessment of muscle-fat content in phantoms, healthy volunteers and patients with achillodynia: comparison to visual assessment of calf muscle quality. Eur Radiol. 2014;24(6):1366-75.
-
27Nicholson CW, Berlet GC, Lee TH. Prediction of the success of nonoperative treatment of insertional Achilles tendinosis based on MRI. Foot Ankle Int. 2007;28(4):472-7.
-
28Van Schie HT, de Vos RJ, de Jonge S, et al. Ultrasonographic tissue characterisation of human Achilles tendons: quantification of tendon structure through a novel non-invasive approach. Br J Sports Med. 2010;44(16):1153-9.
-
29Shaikh Z, Perry M, Morrissey D, et al. Achilles tendinopathy in club runners. Int J Sports Med. 2012;33(5):390-4.
-
30Abat F, Alfredson H, Cucchiarini M, et al. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part I: biology, biomechanics, anatomy and an exercise-based approach. J Exp Orthop. 2017 Dec;4(1):18.
-
31Solan M, Davies M. Management of insertional tendinopathy of the Achilles tendon. Foot Ankle Clin 2007;12(4):597-615.
-
32Hennessy MS, Molloy AP, Sturdee SW. Noninsertional Achilles tendinopathy. Foot Ankle Clin. 2007;12(4):617-41.
-
33Knobloch K. Eccentric training in Achilles tendinopathy: is it harmful to tendon microcirculation? Br J Sports Med. 2007;41(6):e2. discussion e2.
-
34McCormack JR, Underwood FB, Slaven EJ, Cappaert TA. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8(3):230-7.
-
35Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med. 2011;39(3):607-13.
-
36Couppe C, Svensson RB, Silbernagel KG, Langberg H, Magnusson SP. Eccentric or concentric exercises for the treatment of tendinopathies? J Orthop Sports Phys Ther. 2015;45(11):1-25.
-
37Fahlstrom M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):327-33.
-
38Jonsson P, Alfredson H, Sunding K, Fahlstrom M, Cook J. New regimen for eccentric calf-muscle training in patients with chronic insertional Achilles tendinopathy: results of a pilot study. Br J Sports Med. 2008;42(9):746-9.
-
39Lee JY, Yoon K, Yi Y, et al. Long-term outcome and factors affecting prognosis of extracorporeal shockwave therapy for chronic refractory Achilles tendinopathy. Ann Rehabil Med. 2017;41(1):42-50
-
40Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional Achilles tendinopathy. A randomized, controlled trial. J Bone Joint Surg Am. 2008;90(1):52-61.
-
41Taylor J, Dunkerley S, Silver D, et al. Extracorporeal shock-wave therapy (ESWT) for refractory Achilles tendinopathy: a prospective audit with 2-year follow up. Foot (Edinb). 2016;26:23-9.
-
42Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37(3):463-70.
-
43Furia JP. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34(5):733-40.
-
44Wei M, Liu Y, Li Z, Wang Z. Comparison of clinical efficacy among endoscopy-assisted radiofrequency ablation, extracorporeal shockwaves, and eccentric exercises in treatment of insertional Achilles tendinosis. J Am Podiatr Med Assoc. 2017;107(1):11-6.
-
45Chimenti RL, Bucklin M, Kelly M, et al. Insertional Achilles tendinopathy associated with altered transverse compressive and axial tensile strain during ankle dorsiflexion. J Orthop Res. 2017;35(4):910-5.
-
46Chimenti RL, Flemister AS, Ketz J, et al. Ultrasound strain mapping of Achilles tendon compressive strain patterns during dorsiflexion. J Biomech. 2016;49(1):39-44.
-
47Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751-67.
-
48Kearney RS, Parsons N, Metcalfe D, Costa ML. Injection therapies for Achilles tendinopathy. Cochrane Database Syst Rev. 2015;5:CD010960.
-
49Ohberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain—Results of a pilot study. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):339-43.
-
50Crescibene A, Napolitano M, Sbano R, Costabile E, Almolla H. Infiltration of autologous growth factors in chronic tendinopathies. J Blood Transfusion. 2015;2015:924380.
-
51Di Matteo B, Filardo G, Kon E, Marcacci M. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy—a systematic review. Musculoskelet Surg. 2015;99(1):1-9.
-
52O’Malley MJ. PRP Shows Potential For Treating Achilles Tendinosis. New Orleans, LA: American Academy of Orthopaedic Surgeons; 2010.
-
53Redler LH, Thompson SA, Hsu SH, Ahmad CS, Levine WN. Platelet-rich plasma therapy: a systematic literature review and evidence for clinical use. Phys Sportsmed. 2011;39(1):42-51.
-
54Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P. The role of platelets in the treatment of Achilles tendon injuries. J Orthop Res. 2013;31(1):111-8.
-
55Salini V, Vanni D, Pantalone A, Abate M. Platelet rich plasma therapy in non-insertional Achilles tendinopathy: the efficacy is reduced in 60-years old people compared to young and middle-age individuals. Front Aging Neurosci. 2015;7:228.
-
56Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. 2012;33(5):379-85.
-
57Yeo A, Kendall N, Jayaraman S. Ultrasound-guided dry needling with percutaneous paratenon decompression for chronic Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2016 Jul;24(7):2112-8.
-
58Irwin TA. Current concepts review: insertional Achilles tendinopathy. Foot Ankle Int. 2010;31(10):933-9.
-
59Abat F, Alfredson H, Cucchiarini M, et al. Current trends in tendinopathy: consensus of the ESSKA Basic Science Committee. Part II: treatment options. J Exp Orthop. 2018 Sep 24;5(1):38.
-
60Van Dijk CN, van Dyk GE, Scholten PE, Kort NP. Endoscopic calcaneoplasty. Am J Sports Med. 2001;29(2):185-9.
-
61Vega J, Baduell A, Malagelada F, Allmendinger J, Dalmau-Pastor M. Endoscopic Achilles Tendon Augmentation With Suture Anchors After Calcaneal Exostectomy in Haglund Syndrome. Foot Ankle Int. 2018 May;39(5):551-9.
-
62Carmont MR, Maffulli N. Management of insertional Achilles tendinopathy through a Cincinnati incision. BMC musculoskeletal disorders. BMC Musculoskelet Disord. 2007;8:82.
-
63Shakked RJ, Raikin SM. Insertional tendinopathy of the Achilles: debridement, primary repair, and when to augment. Foot Ankle Clin. 2017;22(4):761-80.
-
64Barg A, Ludwig T. Surgical Strategies for the Treatment of Insertional Achilles Tendinopathy. Foot Ankle Clin. 2019 Sep;24(3):533-59.
-
65Greenhagen RM, Shinabarger AB, Pearson KT, Burns PR. Intermediate and long-term outcomes of the suture bridge technique for the management of insertional Achilles tendinopathy. Foot Ankle Spec. 2013;6(3):185-90.
-
66Brady PC, Arrigoni P, Burkhart SS. Evaluation of residual rotator cuff defects after in vivo single- versus double-row rotator cuff repairs. Arthroscopy. 2006;22(10):1070-5.
-
67Kim DH, Elattrache NS, Tibone JE, et al. Biomechanical comparison of a single row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-14.
-
68Ma CB, Comerford L, Wilson J, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-10.
-
69Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-9.
-
70Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon-bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-74.
-
71Beitzel K, Mazzocca AD, Obopilwe E, et al. Biomechanical properties of doubleand single-row suture anchor repair for surgical treatment of insertional Achilles tendinopathy. Am J Sports Med. 2013;41(7):1642-8.
-
72Pilson H, Brown P, Stitzel J, et al. Single-row versus double-row repair of the distal Achilles tendon: a biomechanical comparison. J Foot Ankle Surg. 2012;51(6):762-6.
-
73Andersson G, Backman LJ, Christensen J, et al. Nerve distributions in insertional Achilles tendinopathy - a comparison of bone, bursae and tendon. Histol Histopathol. 2017;32(3):263-70.
-
74Nunley JA, Ruskin G, Horst F. Long-term clinical outcomes following the central incision technique for insertional Achilles tendinopathy. Foot Ankle Int. 2011;32(9):850-5.
-
75Hunt KJ, Cohen BE, Davis WH, et al. Surgical treatment of insertional Achilles tendinopathy with or without flexor hallucis longus tendon transfer: a prospective, randomized study. Foot Ankle Int. 2015;36(9):998-1005.
-
76López-Capdevila L Santamaría Fumas A, Domínguez Sevilla A, et al. Osteotomía calcánea con cuña de sustracción dorsal como tratamiento quirúrgico en la tendinopatía insercional de Aquiles. Rev Esp Cir Ortop Traumatol. 2020;64(1):22-7.
-
77Tourne Y, Baray AL, Barthelemy R, et al. The Zadek calcaneal osteotomy in Haglund’s syndrome of the heel: Clinical results and a radiographic analysis to explain its efficacy. Foot Ankle Surg. 2021 Feb 20;S1268-7731(21)00027-8.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Tendon injury: from eternally forgotten to trendy disease
- Physiology and mechanobiology of tendon and muscle tissue
- Patellar tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Pathophysiology, diagnosis and treatment of Achilles tendinopathy
- Supraspinatus tendinopathy: diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives
- Results of arthroscopic gluteus medius tendon repair in patients with trochanteric pain syndrome. Case series
- Update on the diagnosis and treatment of quadriceps muscle injuries
- Management of triceps surae muscle injuries in young adult and middle-aged athletes: a narrative literature review
- Insertional tendinopathy of the Achilles tendon. Treatment from start to finish
- Inverted cyclops
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.