Perioperative analgesia in arthroscopic surgery of the wrist and hand
Analgesia perioperatoria en cirugía artroscópica de muñeca y mano
Resumen:
Proporcionar una analgesia eficaz es esencial para la recuperación óptima del paciente después de una cirugía. Aunque es sabido que diferentes procedimientos quirúrgicos requieren enfoques específicos para el manejo del dolor, siguen sin existir estudios ni guías para el manejo específico del dolor en los procedimientos artroscópicos de muñeca.
Entre las estrategias terapéuticas de control del dolor postoperatorio destaca la analgesia multimodal, que puede aplicarse en todas las etapas perioperatorias.
Dentro del manejo preoperatorio, no hay estudios disponibles que demuestren si existe o no superioridad de la analgesia administrada antes de una artroscopia de muñeca. En el manejo intraoperatorio, la elección de anestesia general, bloqueos regionales periféricos, bombas de infusión continua, infiltraciones periarticulares, infiltraciones locales y/o la cirugía con el paciente despierto y sin isquemia (wide awake local anesthesia no tourniquet –WALANT–) depende de las características del paciente, de las patologías asociadas y de la localización y la duración estimada del procedimiento específico. La mayoría de los autores siguen recomendando el uso del bloqueo anestésico regional (supraclavicular, infraclavicular, axilar) para la analgesia en pacientes sometidos a artroscopia de muñeca.
Los analgésicos orales son los pilares del control analgésico una vez que el paciente sale del hospital. Después de este tipo de cirugías, generalmente se utiliza una combinación de medicamentos orales entre los que se incluyen el paracetamol, antiinflamatorios no esteroideos (AINE) y combinaciones de ambos con distintos opioides menores. De ellos se conoce la posología, pero no cuánto tiempo se deben administrar, ni cuál es la mejor y más efectiva combinación.
A corto plazo, se deberían realizar estudios y elaborar guías específicas para el manejo del dolor en los procedimientos artroscópicos de muñeca y mano, con el fin mejorar la recuperación de los pacientes y evitar las negativas consecuencias de un mal control posquirúrgico del dolor.
Abstract:
Effective analgesia is essential for optimum patient recovery after surgery. Although different surgical procedures require specific pain management strategies, studies or guides for the specific management of pain in arthroscopic procedures of the wrist remain lacking.
Among the different postoperative pain management strategies, mention must be made of multimodal analgesia, which can be applied in all the perioperative stages.
In relation to preoperative management, no studies to date have confirmed or discarded the superiority of analgesia administered before wrist arthroscopy. In intraoperative management, the choice of general anaesthesia, peripheral regional blocks, continuous infusion pumps, periarticular infiltrations, local infiltrations and/or wide awake local anaesthesia with no tourniquet (WALANT) depends on the patient characteristics, the associated disease conditions, and the location and estimated duration of the specific procedure involved. Most authors continue to recommend regional anaesthetic block (supraclavicular, infraclavicular, axillary) for analgesia in patients subjected to wrist arthroscopy.
Oral analgesics are the cornerstone of pain control once the patient leaves the hospital. Following surgeries of this kind, use is generally made of a combination of oral drugs including paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs) and combinations of both with different mild opioids. The corresponding dosing specifications are known, but it is not clear for how long these medications are to be administered, or which is the best and most effective combination.
Studies are needed and specific guides should be developed for pain management in arthroscopic procedures of the wrist and hand, in order to improve patient recovery and avoid the negative consequences of poor postoperative pain control.
Introduction
Arthroscopic exploration of the wrist has been performed for decades, though arthroscopic surgical techniques involving the wrist are a relatively recent development. A number of factors have favoured these advances, including improvement of the anaesthetic techniques and new analgesic management approaches. Nevertheless, adequate postoperative pain control remains a problem for patients and a challenge for hand surgeons, and there is still a lack of studies and guides on the specific management of pain in these arthroscopic procedures.
Since there are no relevant publications on the perioperative management of pain in arthroscopic surgery of the hand and wrist, the present literature review was carried out to analyse perioperative analgesia in upper limb surgery, seeking to adapt the conclusions drawn to the context of procedures of this kind.
Perioperative pain in arthroscopic surgery of the wrist and hand
Postoperative pain remains one of the main complaints of patients after surgery (both ambulatory and operations requiring admission). It is known that approximately one-third of all patients experience moderate to intense postoperative pain(1,2), and that the latter is associated to an increased number of visits to the emergency rooms and to clinics(3), and even to the development of chronic pain(4). Furthermore, in elderly patients, such pain has been associated to cognitive impairment, an increased risk of dementia, memory loss, and diminished functional activity(2). Considering these potential effects upon patient quality of life, it is clear that the surgical strategy must contemplate correct perioperative pain management.
Most if not all arthroscopic procedures of the hand and wrist can be performed on an ambulatory basis or involving a hospital stay of only a few hours. Even though these are minimally invasive procedures, they would not be possible without adequate pain control. Although in recent years there have been great advances in the recommendations for pain management specifically adapted to each surgical procedure (Procedure-Specific Postoperative Pain Management [PROSPECT]), thanks to initiatives such as that of the European Society of Regional Anaesthesia and Pain Therapy (ESRA), adequate postoperative pain control remains a problem for patients and surgeons, and we still lack specific guides for the management of pain in these procedures — despite the fact that pain control is known to afford a more comfortable and efficient clinical course during rehabilitation, and improves patient satisfaction and the surgical outcomes(5).
A number of strategies are available for offering patients good pain control. In this regard, an important option, due to its known efficiency, is multimodal analgesia, which involves the combination of different anaesthetic agents that act via different mechanisms (e.g. opioids, non-steroidal anti-inflammatory drugs [NSAIDs] and local anaesthetics) with the purpose of securing additive or synergic analgesia, using lower analgesic drug doses and with fewer side effects. Multimodal analgesia not only lowers pain intensity but also reduces the required amounts of analgesic drugs after surgery(6).
Pain management begins in the preoperative stage, providing patients with adequate information, resolving their doubts, and explaining both the surgical and the postsurgical process. An effective postoperative analgesia strategy starts with intraoperative anaesthesia, since it has been shown to be able to affect the level and perception of pain after the operation. The choice of general anaesthesia, peripheral regional blocks, continuous infusion pumps, periarticular infiltrations, local infiltrations and/or wide awake local anaesthesia with no tourniquet (WALANT) depends on the patient characteristics, the associated disease conditions, and the location and estimated duration of the specific procedure involved. Postoperative analgesia begins in the recovery room or postanaesthesia care unit, and includes cryotherapy, oral and injectable drugs, transdermal patches and selective regional blocks. The combination of these methods can condition both the duration of hospital stay and the level of pain control in the first hours after disappearance of the sedative effect. However, perhaps the most challenging element in the analgesic strategy is the choice of an adequate home medication regimen — since excessive medication can produce important side effects with a significant impact upon patient function, while inadequate pain control increases patient morbidity and suffering.
Preoperative management
There is controversy regarding the appropriate moment for starting analgesic therapy.
In order to understand this controversy, it is important to understand and distinguish between the concepts of preventive (anticipatory) analgesia, which considers it more important to start nociceptive treatment before the surgical incision, and precautionary analgesia, which encompasses all the perioperative efforts to lessen pain and opioid consumption(7). Although important review studies such as that published by Moiniche et al.(8), have reported no superiority of analgesia administered before surgery, other studies have shown that the pre- and postoperative use of NSAIDs, including ketorolac, ibuprofen and celecoxib, significantly reduces postoperative pain(9,10,11). On the other hand, no superiority has been observed for paracetamol administered before the incision, though erroneously a recent literature review by Neumeister et al.(12) has attributed it with a 20% decrease in postoperative morphine use when administered 24 hours before surgery. With regard to the concrete setting of arthroscopic surgery of the wrist and hand, no studies are available in support of one body of evidence or the other.
Intraoperative management
The basic elements guiding the intraoperative anaesthesia plan should include multimodal techniques, optimum opioid use, and the anaesthesia most useful in every sense for the patient. This anaesthesia plan includes inhalational, intravenous or regional techniques (peripheral or neuroaxial). The anaesthetist must provide correct intraoperative care and make sure that this combination of techniques is the best for the patient throughout the perioperative process(7). The choice of analgesia and anaesthesia during the surgical procedure not only conditions the duration of patient stay in hospital after surgery but can also have a strong influence upon the level of postoperative pain and its control. The choice of general anaesthesia, peripheral regional blocks, continuous infusion pumps, periarticular infiltrations, local infiltrations and/or WALANT depends on the patient characteristics, the associated disease conditions, and the location and estimated duration of the specific procedure involved(1).
General anaesthesia
There is evidence that in other joints of the upper extremity(13,14), regional anaesthesia usually results in shorter recovery times and faster hospital discharge after surgery compared with general anaesthesia.
In relation to the specific case of hand surgery, Chan et al.(15) found regional anaesthesia to be associated to more favourable patient recovery than general anaesthesia, requiring less nursing care in the recovery ward, and allowing earlier hospital discharge. These findings were confirmed again some years later by McCartney et al.(16). However, Ketonis et al.(1) recorded no differences versus general anaesthesia in the level of pain between the first postoperative day and up to 14 days after surgery.
Peripheral regional blocks
The use of regional anaesthesia may be one of the most powerful tools for anaesthetists in dealing with intense postoperative pain. It has been shown to improve postoperative analgesia and patient satisfaction(17). Single-injection plexus blocks are currently the most widely used option for regional anaesthesia in surgery of the hand and wrist. They involve the injection of a local anaesthetic into a concrete zone of the brachial plexus, affording an analgesic effect during 12-24 hours. The benefits are multiple and include improved clinical, economic and human outcomes. These techniques have been associated with improved postoperative pain control and a decreased use of opioids in many surgical procedures(18), though in our context this is not a major concern, due to their limited use. By decreasing the need for opioids, they can lower the risk of postoperative nausea and vomiting, alterations of mental state and pruritus, thereby reducing hospital resource utilisation and facilitating hospital discharge(19), as well as improving patient recovery and satisfaction(20). In the case of arthroscopic procedures of the hand and wrist, use can be made of supraclavicular, infraclavicular or axillary blocks.
Supraclavicular brachial plexus block
Supraclavicular brachial plexus block is performed at the level of the anterior and posterior divisions of the trunks of the brachial plexus. This block affords complete and reliable anaesthesia of the upper extremity below the middle third of the arm. It can be used for operations of the distal humerus, elbow, forearm, wrist or hand. The typical local anaesthetic dose is 20-25 ml of ropivacaine or bupivacaine at a concentration of 0.5%. In contrast to interscalene blocks, which are more widely used and indicated in surgery of the shoulder, supraclavicular block is associated with a lesser incidence of phrenic nerve block (C3-C5) and secondary ipsilateral hemidiaphragmatic paresis. However, caution is required when considering this type of block in patients with pre-existing lung disease, since they may be unable to tolerate any decrease in pulmonary function(21).
Infraclavicular brachial plexus block
Infraclavicular brachial plexus block is targeted to the brachial plexus at cord level, before emergence of the axillary and musculocutaneous nerve, affording anaesthesia of the upper extremity below the middle third of the arm. This type of block is very appropriate for procedures involving the arm, elbow, forearm, wrist and hand. The typical local anaesthetic dose is 20-30 ml of 0.5% ropivacaine. In comparison with interscalene and supraclavicular blocks, infraclavicular block is associated with a very low incidence of phrenic nerve block (C3-C5) and secondary ipsilateral hemidiaphragmatic paresis. A possible complication, however, is pneumothorax if the needle is advanced too far in depth(21).
Axillary brachial plexus block
Axillary block (Figure 1) is targeted to the terminal branches of the brachial plexus. It can be used in operations of the elbow, forearm, wrist and hand, though it is typically necessary to combine a musculocutaneous nerve block in order to secure analgesia of the entire forearm. The typical local anaesthetic dose is 20-30 ml of 0.5% ropivacaine. Paralysis of the phrenic nerve has not been reported with axillary block; this technique is therefore an excellent option in patients with a history of severe lung disease. In addition, axillary block is a reasonable option in anticoagulated patients, because any inadvertent haematoma can be quickly and widely compressed(21).
The potential risks of peripheral nerve blocks, independently of the technique or location of the block, include vascular puncture and bleeding, nerve damage and systemic toxicity caused by the local anaesthetics. The neurological complications are particularly relevant, since the symptoms may last for weeks or even months after surgery. Patients tend to describe these problems as tingling sensation, pain on applying pressure to the puncture zone, or pricking sensation(22,23). The signs and symptoms of systemic toxicity due to local anaesthetics are dose dependent and range from a metallic taste, tinnitus and perioral numbness to seizures, cardiac arrest and death(24).
Due to the great benefits demonstrated by peripheral nerve blocks in practice, their use has grown in recent decades, with technical advances including the use of ultrasound guidance, which has significantly improved safety, reducing the incidence of vascular puncture(25), and allowing more precise application of the blocks with lesser volumes of anaesthetic(1). Another development has been the shift from single injections of local anaesthetic to continuous anaesthetic infusion using a perineural catheter.
The main limitation of single injection block is the short duration of action of most of the local anaesthetics. Blocks of this kind are therefore very adequate for surgical procedures in which the postoperative pain is not expected to last more than 12-24 hours — since otherwise the patients would be at risk of suffering pain secondary to a significant rebound effect after discharge. The administration of larger volumes or greater concentrations of anaesthetics is possible and can increase the duration of block, though it also increases the risk of motor block and of systemic toxicity of the local anaesthetic(18,26). In contrast to single injections, the administration of local anaesthetics as a continuous infusion allows for significantly longer analgesia, with lower pain and a lesser need for opioid use after surgery(27). Its availability has made it possible for selected patients to be discharged with an ambulatory infusion pump instead of remaining in hospital or receiving other oral analgesics at home. Adequate patient selection, monitoring, and instruction on correct handling and extraction of the pump is important to ensure good use and efficacy. Patients not amenable to the use of this kind of technique include those with known kidney and liver failure, heart and/or lung disease(28), altered mental state or psychosocial problems(29), the impossibility of being contacted after discharge or of reporting to a medical centre in the event of an emergency(26), and patients who are not willing to accept the responsibility of managing the pump(28). Before discharge, the patients are to be instructed on the use, functioning and care of the catheter and the dressing(18,29). Another important aspect to be taken into account is that the costs (referred to both money and time) associated with blocks of this kind are significant and should be duly evaluated by the physicians and hospitals that use such techniques.
The incidence of complications with continuous infusion blocks is largely dependent on the insertion technique and block zone involved(18). The minor complications include migration of the catheter(30) (in up to 25% of all cases), obstruction, and fluid leakage in the catheter zone(31). Although high catheter bacterial colonisation rates have been described, clinically relevant infections are rare(31). The catheter colonisation risk factors include catheter placement for over 48 hours, diabetes, and the administration of antibiotics during the month prior to surgery(32).
Consequently, although continuous infusion catheters eliminate the main limitation of single-injection nerve blocks, they have introduced a new series of difficulties that complicate their routine application.
New continuous infusion catheter modalities are thus needed to minimise the risks of complications and costs, with a view to spreading the use of this type of technique.
Local and periarticular infiltrations and WALANT
Encouraged by the studies of Rolf et al.(33,34), demonstrating the successful use of local anaesthetic injections in knee and ankle arthroscopy portals, hand surgeons transferred the experience to arthroscopy of the wrist and developed the technique, opening new perspectives that have expanded over time. In contrast to surgery of the knee, the advantage in the case of wrist arthroscopy is that sedation is not necessary to achieve good results, since voluntary muscle spasms might not be so critical(35). Likewise through experience gained with arthroscopy of the knee, Karaoglu et al.(36) found that bleeding in arthroscopy mainly comes from the incision zone of the portals. Once it has also been evidenced to be safe to inject adrenaline — a potent vasoconstrictor — into the hand and fingers(37,38), with the injection of local anaesthetic mixed with adrenaline in the portal, clear vision can be achieved without causing haemodynamic changes or ischaemia or necrosis at finger level.
Among the advantages of the WALANT technique, and in the same way as in peripheral regional blocks, mention must be made of the fact that interaction can be established during arthroscopy between the patient and the surgeon. This is advantageous for both of them, since for some patients the possibility of personally witnessing the lesion repair process strengthens their confidence in the surgeon and encourages them to adhere to postoperative rehabilitation(39). Furthermore, although the use of the WALANT technique has not completely eliminated the risk of iatrogenic effects upon the tendons, it does allow immediate evaluation of movement of the fingers intraoperatively and facilitates quick treatment of any possible complications.
The key to success with this technique is the effective application of local anaesthesia in the location of the portal. This requires detailed knowledge of the surface anatomy and great skill in creating the portals. In order to avoid secondary movement of the skin, some authors recommend preparation, placement and fixation of the extremity with the required traction, before infiltrating the local anaesthetic. In contrast to open surgical procedures with WALANT, where a generous volume of local anaesthetic is generally advised, with a considerable waiting time, in the case of arthroscopy we only need a small volume of local anaesthetic and a minimum waiting time for anaesthesia in the portal to become effective(39,40). The type of local anaesthetic and the doses used vary among the different studies(35,39,41,42). Hagert and Lalonde(43) use 20 ml of a solution of 1% lidocaine (10 mg/ml) with adrenaline (5 mg/ml) and 2 ml of sodium bicarbonate (50 mg/ml) for infiltration of the dorsal zone of the wrist, adding another 5 ml of 1% lidocaine with adrenaline within the radiocarpal joint. These authors recommend administration of the anaesthesia at least 30 minutes before surgery, to allow enough time to establish the desired anaesthetic level. However, those authors that use lidocaine as local anaesthetic at a greater concentration (2%) — which has a much faster onset of effect — start surgery almost immediately after its administration(35). Likewise, some authors consider that the intraarticular infiltration of anaesthetic is not necessary in most cases, unless a considerable intraarticular treatment procedure is expected(35). If so, the additional injection of 4 ml of local anaesthetic with adrenaline into the joint facilitates adequate anaesthesia(39). This mixture also affords effective local haemostasis in the joint capsule and synovial membrane, and thus avoids the routine use of a tourniquet(35). If necessary, the local anaesthetic in the portals can be combined with WALANT infiltration techniques for open surgery.
The potential risks of anaesthesia injected in the portals include systemic effects of the local anaesthetic if it enters the general circulation, the vasoconstrictive effect of adrenaline, and the theoretical risk of permanent cartilage damage when the local anaesthetic is injected into the joint. The concern about causing permanent cartilage damage comes from studies on the viability of chondrocytes in contact with these amide based drugs, such as lidocaine, bupivacaine or levobupivacaine, conducted in vitro and in laboratory animals(44,45,46,47). However, no direct causal relationship has been evidenced between local anaesthetic infusion and chondrolysis in clinical studies(35). Likewise, to date, no studies have been made on the effect of local anaesthetics upon joint cartilage in arthroscopy of the wrist.
Situations of known hypersensitivity to the local anaesthetics used and adrenaline are absolute contraindications to the use of this technique. The existence of heart disease has been described in the literature as a relative contraindication, because patients of this kind may be particularly sensitive to adrenaline(48). It is not considered to be an adequate technique in procedures that involve a lot of work on bone. Lastly, the technique is not indicated in young and immature patients, individuals with severe anxiety, mental disability, uncontrolled psychiatric disease or a low pain threshold(35).
The minimally invasive nature of arthroscopy makes it particularly suitable for the WALANT technique. However, in contrast to the block procedures, it does not offer good pain control after surgery. Studies such as those published by Hansen and Jakobsen(49) have shown that the intraarticular injection of 5 ml of 0.5% bupivacaine after arthroscopy of the wrist reduces pain and the need for analgesics only during the first two hours. Anaesthetic block is therefore recommended for arthroscopy of the wrist(41).
Postoperative management
Recovery ward
The effective management of pain in the recovery ward can have a strong impact upon patient satisfaction and well-being, the duration of admission and the postoperative course once the patient returns home after surgery. Morphine and fentanyl are the drugs most frequently administered to ambulatory patients during the first stage of recovery. The recommendation of fentanyl is due to its very rapid onset of action, which allows faster pain control and potentially reduces the total opioid dosage used for this purpose and the potential related side effects(1). In a randomised prospective trial, Claxton et al.(50) concluded that combining morphine with fentanyl reduces the side effects, facilitating hospital discharge, and producing fewer complications after discharge due to lowering of the morphine doses needed to control the pain.
Home analgesia
Extension of the multimodal analgesia strategy to the postoperative period has afforded great benefit in terms of pain control(7). Oral analgesics are the cornerstone of pain control once the patient leaves the hospital. The prescribed drugs should allow patients to perform their activities of daily living, with minimum side effects and no interferences with the healing process. In addition, they should be easy to handle on the part of the patients(1). In the context of injury, the prostaglandins act as inflammatory mediators that sensitise the nociceptors of the pain pathway at both central and peripheral nervous system level. Modulation of the inflammatory pathway is therefore a key objective for the control of postoperative pain(12). Thus, following ambulatory arthroscopic surgery of the wrist, use is generally made of a combination of oral drugs that reduce inflammation and prostaglandin production, including paracetamol, NSAIDs and combinations of both with different mild opioids. The dosing specifications of these drugs are known, but there is no firm evidence as to how long they should be administered(7). Likewise, there is no evidence that any concrete drug offers specific effectiveness in these procedures.
Paracetamol
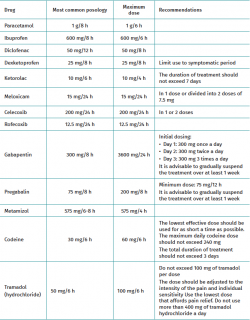
Paracetamol is one of the most widely used analgesics worldwide. Analgesia using paracetamol is known to not have a very high ceiling, though it may be adequate for the management of mild to moderate pain. The drug is effective, safe, inexpensive, and offers a favourable adverse effects profile(51). Its mechanism of action is little known, however. There is some evidence that paracetamol exerts a central antinociceptive effect, and it also may prevent prostaglandin production at cellular level. In contrast to the NSAIDs, paracetamol does not irritate the gastric mucosa, does not affect platelet function, and does not cause renal failure; it is therefore a very versatile drug(1). The most commonly used dose is 1 g every 8 hours, though a maximum dose of 1 g every 6 hours can be administered if necessary (Table 1).
Non-steroidal anti-inflammatory drugs (ibuprofen, diclofenac, dexketoprofen, ketorolac, meloxicam)
The NSAIDs form part of most pain management protocols in ambulatory surgery, including arthroscopy of the wrist. Their anti-inflammatory effects not only afford pain relief but also help to reduce local oedema and minimise the use of other more potent drugs. In the absence of contraindications, the NSAIDs are considered to be the drugs of choice following most ambulatory surgeries(52). Although there are reports indicating that the NSAIDs exert some central action(53), the accepted mechanism of action remains the attenuation of prostaglandin synthesis through the inhibition of cyclooxygenase (COX)(54). One of the main concerns with NSAID use continues to be the gastrointestinal toxicity of these drugs. This led to the discovery of the two COX isoenzymes and to the development of selective COX-2 inhibitors, which the World Health Organisation (WHO) defined as a new NSAID subclass: the coxibs.
The coxibs are equipotent to the traditional NSAIDs in terms of analgesic efficacy(55), and although there is still controversy regarding their safety and concerns about an increased risk of myocardial infarction, all the studies indicate that there appear to be no clear differences in cardiovascular risk between the currently available coxibs and the non-selective NSAIDs when used at the recommended doses(1,56). Another concern with NSAID use is the theoretical associated risk of causing bone consolidation problems, though the evidence of such an effect is not strong enough to contraindicate NSAID use in orthopaedic procedures(57). The most commonly used NSAIDs in our setting, with their most commonly used doses, their maximum doses and some recommendations are described in Table 1.
Gabapentinoids
The gabapentinoids, such as gabapentin and pregabalin, block the voltage-dependent calcium channels and modulate excitatory neurotransmitter release(9), reducing hypersensitivity in the neurons of the dorsal horn of the spinal cord(57). They are associated to a reduction of postoperative pain and a lesser risk of progression towards chronic pain by mitigating central and peripheral sensitisation(12). Both drugs have demonstrated efficacy when administered both after and before the operation, though there is no agreement as to the recommended perioperative dose. Some studies advise starting treatment two weeks before surgery(58), while others recommend a single large bolus dose one or two hours before the operation. In turn, other authors limit their use to the postoperative period(17). The most commonly used gabapentin and pregabalin doses, together with the recommendations and maximum doses, are reported in Table 1.
Metamizol
Metamizol is a non-opioid analgesic with antipyretic, antispasmodic and anti-inflammatory effects(59). The site and mechanism of action of the drug are not fully clear. However, it appears to exert combined central and peripheral action(60). Metamizol affords good analgesia (it is considered to be more potent than paracetamol), with a low incidence of side effects, and its practically 100% bioavailability makes the oral route an adequate way to administer the drug in most cases(61). The most commonly used dose is 575 mg every 6-8 hours. A maximum dose of 575 mg every four hours can be prescribed if necessary (Table 1).
Mild opioids (codeine and tramadol)
Codeine and tramadol are the most frequently used mild opioids via the oral route.
Codeine in itself lacks analgesic activity. The efficacy of this drug is based on its metabolic transformation into morphine (2-10% of the administered dose). The enzyme in charge of this conversion into morphine (CYP isoenzyme) is missing in approximately 10% of all Caucasians(62); this may represent a major inconvenience with its use in our setting.
The recommended codeine dose is 30 mg every 6 hours. The lowest effective dose should be used for as short a time as possible. The maximum daily dose should not exceed 240 mg, and the total duration of treatment should be limited to three days (Table 1).
Tramadol acts as an opioid agonist, and in a combined form as a serotonin and noradrenaline reuptake inhibitor(63). Its oral administration is generally in the form of hydrochloride, and its adverse effects profile is different from that of other opioids. The risk of respiratory depression is significantly lower at analgesia equivalent doses(64). The drug moreover has limited effects upon gastrointestinal motor function and causes less constipation than morphine(65,66). The main inconvenience of tramadol is a high incidence of nausea and vomiting, which implies greater patient dissatisfaction(66). The recommended tramadol hydrochloride dose is 50-100 mg every 4-6 hours. An amount of 100 mg of tramadol per dose should not be exceeded. The drug normally should be administered at the lowest dose that affords pain relief. No more than 400 mg of tramadol hydrochloride a day should be used (Table 1).
Drug formulations combining tramadol and paracetamol at different doses are very common. The most frequently prescribed pharmacological presentation is 37.5 mg/325 mg, respectively, with a usual dose of two tablets every 8 hours, and a maximum of two tablets every 6 hours.
A study from 2007 comparing the analgesic effects of tramadol, metamizol and paracetamol in patients subjected to ambulatory surgery of the hand found that none of the three drugs afforded effective analgesia in all patients when administered isolatedly(66). The percentage of patients requiring rescue treatment with another opioid at home was 42% in the case of paracetamol, 31% in the case of metamizol and 23% in the case of tramadol. However, tramadol was associated to an increased frequency and severity of adverse effects such as nausea and dizziness, and thus produced greater patient dissatisfaction.
Taking into account that no drug combination has been found to be superior to another, patient instructions and information about what to except, the way to control the pain, and how to use the prescribed medication remain crucial aspects(1). A patient who is implicated and well informed about the treatment plan, the options, objectives and expectations referred to pain after surgery will be more satisfied than a patient who is not.
Physical measures
In addition to pharmacological interventions, we also must consider the adoption of physical measures in the postoperative management of pain. These include cryotherapy, transcutaneous electrical nerve stimulation (TENS), acupuncture, massages and the local application of warmth. All these measures are safe and pose practically no risks for the patient.
Transcutaneous electrical nerve stimulation consists of the application of an electric current through surface electrodes affixed to the skin, in order to eliminate the pain. This technique induces hyperstimulation of the sensory fibres that block synaptic transmission of the fibres at spinal cord level. It is believed to activate descending pain inhibitory pathways, reducing the response to pain. The electrodes are generally placed directly on the skin in the operated zone, though some studies have found it useful to also place them at a distance over acupuncture points(12). A review of over 20 randomised trials found TENS to afford a decrease in postoperative analgesia needs of 25%(67).
Cryotherapy involves enveloping the operated wrist with cold pads, cooled air or fluid circulation devices, thereby reducing tissue temperature, oedema and pain. The different studies published to date have obtained variable results, and there is no consensus regarding the benefit of cryotherapy with respect to therapy without cold application, in terms of pain or total analgesic use(12). Likewise, no evidence has been obtained of the usefulness of cryotherapy combined with compression(68).
Lastly, although some studies advocate their application, the evidence on the usefulness of acupuncture and massages in reducing postoperative pain in adults and their effects in terms of a decrease in analgesic use is contradictory — with not enough arguments in favour of recommending their use.
Conclusions
The development of arthroscopic surgical techniques involving the wrist is relatively recent. A number of factors have favoured this progress. Improved anaesthetic techniques and new analgesic approaches have played an important role. However, there still are no studies or guides on the specific management of pain in arthroscopic procedures of the wrist and hand.
With regard to preoperative management, there is controversy regarding the appropriate moment for starting analgesic therapy. No studies to date have confirmed or discarded the superiority of analgesia administered before surgery. With regard to intraoperative management, the anaesthesia plan includes inhalational, intravenous or regional techniques (peripheral or neuroaxial). The choice of anaesthetic technique depends on the patient characteristics, the associated disease conditions, and the location and estimated duration of the specific procedure involved(1).
Of the different techniques, most authors continue to recommend anaesthetic block. Supraclavicular, infraclavicular and axillary blocks are the most appropriate for these surgeries. Among the different postoperative pain management strategies, mention must be made (due to its demonstrated efficiency) of multimodal analgesia, which can be applied in all the perioperative stages. Oral analgesics are the cornerstone of pain control once the patient leaves the hospital. The prescribed drugs should allow patients to perform their activities of daily living, with minimum side effects and no interferences with the healing process. In addition, they should be easy to handle on the part of the patients(1). Lastly, in addition to pharmacological interventions, we also must consider the adoption of physical measures in the postoperative management of pain. Although there is no solid scientific evidence supporting most of them, all such measures are safe and pose practically no risks for the patient.
Since it is known that different surgical procedures require specific pain management approaches, due to the different characteristics of the pain — including its nature (somatic or visceral), location, intensity and duration — and the different consequences of inadequate or inappropriate pain relief, it would be highly recommendable to carry out studies and develop specific guides for the management of pain in arthroscopic procedures of the wrist and hand.
Información del artículo
Cita bibliográfica
Autores
Fernando Polo Simón
Unidad de Cirugía de la Mano y del Miembro Superior. Servicio de Cirugía Ortopédica y Traumatología. Hospital Universitario HM Montepríncipe. Universidad San Pablo CEU. Boadilla del Monte, Madrid.
Servicio de Cirugía Ortopédica y Traumatología. Mutua Universal. Madrid
Víctor Triviño Sánchez-Mayoral
Unidad de Cirugía de la Mano y Microcirugía. Hospital Universitario HM Montepríncipe. Boadilla del Monte, Madrid
Alfonso Carlos Prada Cañizares
Unidad de Cirugía de la Mano y Microcirugía. Hospital Universitario HM Montepríncipe. Boadilla del Monte, Madrid
Hospital Universitario 12 de Octubre, Madrid, España
Pedro José Delgado Serrano
Unidad de Cirugía de la Mano y Microcirugía. Hospital Universitario HM Montepríncipe. Boadilla del Monte, Madrid
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that this research has not involved human or animal experimentation.
Data confidentiality. The authors declare that the protocols of their work centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgements
Thanks are due to Dr. Ignacio Pinazo Osuna and to the nurse Ruth García Carmona, of the Department of Anaesthesia of Hospital Nuestra Señora del Rosario (Madrid, Spain), for their scientific advice and collaboration in the literature search.
Referencias bibliográficas
-
1Ketonis C, Ilyas AM, Liss F. Pain management strategies in hand surgery. Orthop Clin North Am. 2015;46(3):399-408.
-
2Lanitis S, Mimigianni C, Raptis D, et al. The impact of educational status on the postoperative perception of pain. Korean J Pain. 2015;28(4):265-74.
-
3Wu CL, Berenholtz SM, Pronovost PJ, et al. Systematic review and analysis of postdischarge symptoms after outpatient surgery. Anesthesiology. 2002;96(4):994-1003.
-
4Malik OS, Kaye AD, Urman RD. Perioperative hyperalgesia and associated clinical factors. Curr Pain Headache Rep. 2017;21:4.
-
5Warrender WJ, Syed UAM, Hammoud S, et al. Pain management after outpatient shoulder arthroscopy: a systematic review of randomized controlled trials. Am J Sports Med. 2017;45(7):1676-86.
-
6Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth. 2001;13(7):524-39.
-
7Pozek JJ, De Ruyter M, Khan TW. Comprehensive acute pain management in the perioperative surgical home. Anesthesiol Clin. 2018;36(2):295-307.
-
8Moiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology. 2002;96(3):725-41.
-
9Argoff. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477-87.
-
10Huang YM, Wang CM, Wang CT, et al. Perioperative celecoxib administration for pain management after total knee arthroplasty: a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
-
11Giuliani E, Bianchi A, Marcuzzi A, et al. Ibuprofen timing for hand surgery in ambulatory care. Acta Ortop Bras. 2015;23(4):188-91.
-
12Neumeister EL, Beason AM, Thayer JA, et al. Perioperative pain management in hand and upper extremity surgery. Clin Plastic Surg. 2020;47(2):323-34.
-
13D'Alessio JG, Rosemblum M, Shea KP, et al. A retrospective comparison of interscalene block and general anesthesia for ambulatory surgery shoulder arthroscopy. Reg Anesth. 1995;20(1):62-8.
-
14Brown AR, Weiss R, Greenberg C, et al. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
-
15Chan VW, Peng PW, Kaszas Z, et al. A comparative study of general anesthesia, intravenous regional anesthesia, and axillary block for outpatient hand surgery: clinical outcome and cost analysis. Anesth Analg. 2001;93(5):1181-4.
-
16McCartney CJL, Brull R, Chan VWS, et al. Early but not long-term benefit of regional compared with general anesthesia for ambulatory hand surgery. Anesthesiology. 2004;101(2):461-7.
-
17Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-57.
-
18Joshi G, Gandhi K, Shah N, et al. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. Journal Clin Anesth. 2016;35:524-9.
-
19Brummett CM, Williams BA. Additives to local anesthesics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49(4):104-16.
-
20Liu Q, Chelly JE, Williams JP, et al. Impact of peripheral nerve block with low dose local anesthetics on analgesia and functional outcomes following total knee arthroplasty: a retrospective study. Pain Med. 2015;16(5):998-1006.
-
21Ardon AE, Prasad A, McClain RL, et al. Regional anesthesia for ambulatory anesthesiologists. Anesthesiol Clin. 2019;37(2):265-87.
-
22Widmer B, Lustig S, Scholes CJ, et al. Incidence and severity of complications due to femoral nerve blocks performed for knee surgery. Knee. 2013;20:181-5.
-
23Compere V, Rey N, Baert O, et al. Major complications after 400 continuous popliteal sciatic nerve blocks for post-operative analgesia. Acta Anaesthesiol Scand. 2009;53:339-45.
-
24Morau D, Ahem S. Management of local anesthetic toxicity. Int Anesthesiol Clin. 2010;48:117-40.
-
25Abrahams MS, Aziz MF, Fu RF, et al. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2009;102:408-17.
-
26Salinas Fv, Joseph RS. Peripheral nerve blocks for ambulatory surgery. Anesthesiol Clin. 2014;32:341-55.
-
27Binham AE, Fu R, Horn JL, et al. Continuous peripheral nerve block compared with single-injection peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med. 2012;37:583-94.
-
28Aguirre J, Del Moral A, Cobo I, et al. The role of continuous peripheral nerve blocks. Anesthesiol Res Pract. 2012;2012:560879.
-
29Greengrass RA, Nielsen KC. Management of peripheral nerve block catheters at home. Int Anesthesiol Clin. 2005;43:79-87.
-
30Marhofer D, Marhofer P, Triffterer L, et al. Dislocation rates of perineural catheters: a volunteer study. Br J Anaesth. 2013;111:800-6.
-
31Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904-25.
-
32Aveline C, Le Hetet H, Le Roux A, et al. Perineural ultrasound-guided catheter bacterial colonization: a prospective evaluation in 747 cases. Reg Anesth Pain Med. 2011;36:579-84.
-
33Rolf C. Knee arthroscopy under local anaesthesia. Hong Kong J Orthop Surg. 1998;2(2):158-63.
-
34Rolf C, Saro C, Engsträm B, et al. Ankle arthroscopy under local anaesthesia for diagnostic evaluation and treatment. Scand J Med Sci Sports. 1996;6:255-8.
-
35Koo SCJJ, Ho PC. Wrist Arthroscopy Under Portal Site Local Anesthesia Without Tourniquet and Sedation. Hand Clin. 2017;33(4):585-91.
-
36Karaoglu S, Dogru K, Kabak S, et al. Effects of epinephrine in local anesthetic mixtures on hemodynamics and view quality during knee arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2002;10(4):226-8.
-
37Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51(5):755-9.
-
38Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in finger. Plast Reconstr Surg. 2007;119(1):260-6.
-
39Liu B, Yew C, Arshad MS, et al. Wide-awake wrist and small joints arthroscopy of the hand. Hand Clin. 2019;85-92.
-
40Mak MCK, Ho PC, Tse WL, et al. Arthroscopic resection of wrist ganglion arising from the lunotriquetral joint. J Wrist Surg.2013;2:355-8.
-
41Agrawal Y, Russon K, Chakrabarti I, et al. Intra-articular and portal infiltration versus wrist block for analgesia after arthroscopy of the wrist. Bone Joint J. 2015;97-B:1250-6.
-
42Ong MTY, Ho PC, Wong CWY, et al. Wrist arthroscopy under portal site local anesthesia (PSLA) without tourniquet. J Wrist Surg. 2012;1:149-52.
-
43Hagert E, Lalonde DH. Wide-awake wrist arthroscopy and open TFCC repair. J Wrist Surg. 2012;1(01):55-60.
-
44Cobo_Molinos J, Poncela-García M, Marchal-Corrales JA, et al. Effect of levobupivacaine on articular chondrocytes: an in-vitro investigation. Eur J Anaesthesiol. 2014;31:635-9.
-
45Chu CR, Izzo NJ, Coyle CH, et al. The in vitro effects of bupivacaine on articular chondrocytes. J Bone Joint Surg (Br). 2008;90-B:814-20.
-
46Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0,5% bupivacaine or articular cartilage. J Bone Joint Surg (Am). 2010;92-A:599-608.
-
47Dogan N, Erdem AF, Erman Z, et al. The effects of bupivacaine and neostigmine or articular cartilage and synovium in the rabbit knee joint. J Int Med Res. 2005;32(5):513-9.
-
48Lalonde D. Reconstruction of the hand with wide awake surgery. Clin Plast Surg. 2011;38(4):761-9.
-
49Hansen TB, Jacobsen IA. Intra-articular bupivacaine as treatment for postoperative pain after arthroscopy of the wrist. Scand J Plast Reconstr Surg Hand Surg. 2008;42:313-5.
-
50Claxton AR, McGuire G, Chung F, et al. Evaluation of morphine versus fentanyl for postoperative analgesia after ambulatory surgical procedures. Anesth Analg. 1997;84(3):509-14.
-
51Zhang WY, Li Wan Po A. Analgesic efficacy of paracetamol and its combination with codeine and caffeine in surgical pain: a meta-analysis. J Clin Pharm Ther. 1996;21(4):261-82.
-
52Rawal N, Hylander J, Nydahl PA, et al. Survey of postoperative analgesia following ambulatory surgery. Acta Anaesthesiol Scand. 1997;41(8):1017-22.
-
53Yaksh TL, Malmberg AB. Spinal actions of NSAIDS in blocking spinally mediated hyperalgesia: the role of cyclooxygenase products. Agents Actions Suppl. 1993;41:89-100.
-
54Vane. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 1971;231(25):232-5.
-
55Romsing J, Moiniche S. A systematic review of COX-2 inhibitors compared with traditional NSAIDs, or different COX-2 inhibitors for post-operative pain. Acta Anaesthesiol Scand. 2004;48(5):525-46.
-
56Yancey DL, Calamia KT. Use of COX-II inhibitors for hand surgery patients. J Hand Surg Am. 2008;33(10):1909-10.
-
57Bowers MR, Pulos N, Pulos PB, et al. Opioid-sparing pain management in upper extremity surgery. Part 2: surgeon as prescriber. J Hand Surg Am. 2019;44(10):878-82.
-
58Thomas DA, Boominathan P, Goswami J, et al. Perioperative management of patients with addiction to opioid and non-opioid medications. Curr Pain Headache Rep. 2018;22(7):52.
-
59Lorenzetti BB, Ferriera SH. Mode of analgesic action of dipyrone: direct antagonism of inflammatory hyperalgesia. Eur J Pharmacol. 1985;114:375-81.
-
60Carlsson KH, Helmreich J, Jurna I. Comparison of central antinociceptive and analgesic effects of pyrazolone derivatives, metamizol (dipyrone) and aminophenazone (“pyramidon”). Schmerz Pain Douleur. 1986;7:93-100.
-
61Rawal N, Allvin R, Amilon A, et al. Postoperative analgesia at home after ambulatory hand surgery: a controlled comparison of Tramadol, Metamizol, and Paracetamol. Anesth Analg. 2001;92:347-51.
-
62Caraco Y, Sheller J, Wood AJJ. Pharmacogenetic determination of the effects of codeine and prediction of drug interactions. J Pharmacol Exp Ther. 1996;278:1165-74.
-
63Raffa RB, Friderichs E, Reinmann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an "atypical" opioid analgesic. J Pharmacol Exp Ther. 1992;260:275-85.
-
64Tarkkila P, Tuominem M, Lingred L. Comparison of respiratory effects of tramadol and pethidine. Eur J Anaesthesiol. 1998;15:64-8.
-
65Wilder-Smith CH, Bettiga A. The analgesic tramadol has minimal effect on gastrointestinal motor function. Br J Clin Pharmacol. 1997;43:71-5.
-
66Rawal N. Postoperative pain treatment for ambulatory surgery. Best Prac Res Clin Anaesthesiol. 2007;21(1):129-48.
-
67Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7(2):181-8.
-
68Meyer-Marcotty M, Jungling O, Vaske B, et al. Standarized combined cryotherapy and compression using Cryo/Cuff after wrist arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:314-9.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- The importance of pain control
- Learning from pain to make the patient feel better
- Perioperative pain management in arthroscopic surgery of the shoulder
- Perioperative pain management in arthroscopy of the elbow
- Perioperative analgesia in arthroscopic surgery of the wrist and hand
- Perioperative analgesia in arthroscopy of the hip
- Perioperative analgesia in arthroscopic surgery of the knee
- Perioperative pain management in arthroscopy of the ankle
- Postoperative neuropathic pain in traumatology
- Capsaicin 179 mg patch application technique
- Type 3 SLAP: bucket handle tear
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.