Postoperative neuropathic pain in traumatology
Dolor neuropático posquirúrgico en traumatología
Resumen:
El dolor postoperatorio agudo no controlado o mal controlado es un potente predictor de aparición de dolor neuropático crónico posquirúrgico. El dolor crónico postoperatorio persistente es una entidad compleja cuya etiología no está del todo dilucidada, lo que afecta la calidad de vida de los individuos. El dolor crónico después de la cirugía suele ser, al menos en parte, neuropático, requiriendo su tratamiento un enfoque integral: biológico, psicológico y social. El dolor neuropático resultante de un trauma quirúrgico sigue siendo la expresión más común de esta entidad. El dolor neuropático posquirúrgico es un problema creciente debido al aumento y al envejecimiento de la población, a que se realizan cada vez más cirugías y a que se diagnostica más. Para su prevención es fundamental una adecuada analgesia perioperatoria y se recomienda utilizar siempre que sea posible técnicas que eviten el daño nervioso.
A pesar de los esfuerzos por comprender y seleccionar a los pacientes de riesgo, el manejo y la prevención de esta entidad son aún inadecuados.
La prevención es aquí también clave, como en otras facetas de la medicina, aplicándola de forma individualizada a los pacientes de riesgo.
Abstract:
Uncontrolled or poorly controlled acute postoperative pain is a strong predictor of the appearance of chronic neuropathic pain after surgery. Persistent chronic pain after surgery is a complex condition, with an aetiology that has still not been fully clarified, and which affects patient quality of life. Chronic pain after surgery is usually at least in part of a neuropathic nature, and its management requires an integral approach: biological, psychological and social. Neuropathic pain secondary to surgical trauma remains the most common expression of this condition. Postsurgical neuropathic pain is a growing problem due to the ageing of the population, the ever increasing number of surgeries performed, and the fact that the disorder is diagnosed more often. Its prevention requires adequate perioperative analgesia, and it is advisable to use techniques that avoid nerve damage whenever possible.
Despite the efforts to understand and select patients at risk, the management and prevention of this disorder remain inadequate.
As in other areas of Medicine, prevention here is a key aspect, with application on an individualised basis in patients at risk.
Definition
The new International Classification of Diseases (ICD-11) divides chronic pain into 7 groups: chronic primary pain, chronic cancer-related pain, chronic postsurgical pain (CPSP) or posttraumatic pain, chronic neuropathic pain, orofacial pain and headache, chronic visceral pain and chronic musculoskeletal pain.
This has medical-legal implications in distinguishing conditions with similar pathophysiological characteristics but which differ in aetiological terms (e.g. CPSP and chronic posttraumatic pain)(1).
Chronic postsurgical pain was first defined in 1999 by Macrae and Davis(2), and the definition was subsequently expanded by Macrae(3) in 2001 as "pain developing after surgery and which persists for at least two months". Thus, CPSP is understood as pain persisting for over two months after the operation, excluding other causes of pain unrelated to surgery or to some pre-existing problem(3,4).
In 2014, Werner and Kongsgaard(5) updated the definition of CPSP or persistent postsurgical pain: "Pain persisting for at least three months after surgery, that was not present before surgery or which presents characteristics different from or of greater intensity with respect to presurgical pain, located at the surgical site or in a referred area, and excluding other possible causes of the pain (e.g. cancer recurrence, infection)".
If the characteristics of the pain do not differ with respect to the presurgical features, or if its severity decreases due to surgery, the disorder should not be described as CPSP.
Werner(5), thus proposed 5 updating aspects:
- The pain develops following a surgical procedure, or increases in intensity after the surgical procedure.
- The pain must have a duration of at least 3-6 months and significantly affect patient health-related quality of life (HRQoL).
- The pain is a continuation of acute postoperative pain or develops after an asymptomatic period.
- The pain is located in the surgical field, projects to the territory innervated by a nerve located in the surgical field, or is referred to a dermatome (following surgery of visceral or deep somatic tissues).
- Other causes of pain (infection or persistent malignancy in cancer surgery) are to be excluded.
According to the ICD-11, neuropathic pain is perceived as a burning pain or electric current sensation attributable to metabolic, nutritional, infectious, genetic, autoimmune or vasculitic disorders. The pain may manifest spontaneously, without provocation, or may be induced by different harmful or innocuous stimuli. The pain is characteristic of small fibre neuropathy, though even large fibre neuropathy can affect sufficient small fibres to cause pain. Neuropathic pain usually affects the subcutaneous structures and the skin in peripheral (acral) zones. The pain may be constant or intermittent and can be described as a lacerating, burning or cold sensation.
Complex regional pain syndrome (CRPS) manifests following trauma, and is characterised by regional pain, sensory alterations, dysthermia, sudomotor activity, skin colour changes and oedema (link to access this disorder at ICD-11: http://id.who.int/icd/entity/1834504950).
On the other hand, chronic neuropathic pain is described as "long-lasting pain caused by injury or disease of the somatosensory nervous system". The pain may be spontaneous or induced, and represents an exaggerated response to a painful stimulus (hyperalgesia) or a painful reaction to a stimulus which normally does not cause pain (allodynia). The diagnosis of chronic neuropathic pain requires a history of nervous system injury or disease and a distribution of the pain that appears plausible from the neuroanatomical perspective. The negative (e.g. a decrease or loss of sensitivity) and positive sensory signs and symptoms (e.g. allodynia or hyperalgesia) pointing to alterations of the somatosensory nervous system must be consistent with the territory innervated by the affected nervous structure (6) (link to access this disorder at ICD-11: http://id.who.int/icd/entity/1170330671).
The most consistent characteristic associated with the appearance of CPSP is the duration of intense acute postsurgical pain. In this sense, acute postsurgical pain can be of two types: inflammatory pain and neuropathic pain(7). Acute pain can cause central sensitisation, reducing the pain threshold and incrementing the response to harmful stimuli. Thus, the patient may experience both hyperalgesia and allodynia(8).
In the event of damage to one or more nerves during surgery, patients may frequently develop neuropathic pain, with peripheral and central sensitisation phenomena that lead to chronification of the pain, and give rise to chronic postsurgical neuropathic pain(9). In fact, the prevalence of the neuropathic component in cases of CPSP varies according to the type of surgery involved and is dependent upon the probability of surgical iatrogenic nerve damage(10).
Epidemiology
Crombie(11) published the first article on CPSP in 1998, and since then the literature has basically published results referred to different types of surgical procedures.
The surgeries with the greatest incidence of CPSP are associated with intentional or non-intentional nerve damage, such as the amputation of extremities, mastectomy, and posterolateral thoracotomy. The incidence of chronic pain following these surgeries ranges between 60-70%, approximately(12).
The reported incidence of CPSP varies according to the surgical procedure involved and differs among studies — covering a broad range from 5-85%. For example, the reported incidence is 50-85% in the case of the amputation of an extremity, 11-57% following mastectomy, 30-55% after heart surgery, 5-65% after thoracotomy, and 5-63% following inguinal hernia repair(13).
The studies on processes in the field of traumatology report incidences ranging from 50-85% in the case of the amputation of an extremity, or 5-65% after thoracotomy(13).
Although the great majority of patients undergoing surgery receive analgesic treatment of some kind, almost 40% suffer moderate to intense pain in the first 24 hours postsurgery(14). Furthermore, 50-75% do not achieve complete pain relief in the period of time considered to be normal(15).
Fletcher(16), in a study involving European surgical patients, found 11.8% of the subjects to suffer moderate to intense pain, while 2.2% reported intense pain (visual analogue scale [VAS] ≥ 6) at 12 months postsurgery. The prevalence of chronic pain after surgery is estimated to be 10-50%(17). The pain may be intense and functionally limiting one year after surgery, with rates that range from 2-85%(18).
The under-treatment of pain implies short-term morbidity and moreover favours chronification(17).
All this undoubtedly affects patient quality of life, and pain after surgery moreover is considered to be a frequent cause of patient dissatisfaction, independently of the clinical outcome(19).
The study published by Fletcher(16) also found orthopaedic surgery to be associated with an almost three-fold higher risk of moderate to severe CPSP compared with all other procedures, as determined after 12 months.
Localised neuropathic pain (LNP)
It is a peripheral neuropathic pain characterised by a consistent and circumscribed area of maximum pain intensity equivalent in size to no more than a standard DIN-A4 sheet of paper, associated to abnormal skin sensitivity and/or spontaneous symptoms typical of neuropathic pain(20,21).
The International Association for the Study of Pain (IASP) defines LNP as pain caused by a lesion or disorder of the peripheral somatosensory nervous system; the pain is spontaneous or evoked as an enhanced response to a painful stimulus (hyperalgesia) or a painful response to a stimulus that is normally not painful (allodynia) — though the negative (e.g. reduction or loss of sensitivity) and positive sensory symptoms or signs (e.g. allodynia or hyperalgesia) indicating involvement of the peripheral somatosensory nervous system must be consistent with the territory innervated by the affected nervous structure, which in the case of surgery may follow the wound trajectory secondary to local peripheral innervation damage.
The pathophysiological basis of the chronification of neuropathy is explained by the fact that a peripheral stimulus maintained over time induces plastic changes at peripheral and spinal cord level. On the other hand, neuropathic pain incorporates neuroinflammatory components responsible for the increase in peripheral sensitisation area. Sensitisation (peripheral and central) causes a decrease in the pain threshold and an increase in nociceptive information transmission, with a loss of efficacy of the endogenous control systems, associated to central and cortical reorganisation changes.
The prevalence of LNP is 2% in the general population and reaches 8% in patients over 55 years of age(22) — with LNP representing 60% of all presentations of neuropathic pain(23).
Complex regional pain syndrome
Two types of complex regional pain syndrome (CRPS) are currently contemplated:
- Type I CRPS (previously referred to as reflex sympathetic dystrophy). According to the new ICD-11, type I CRPS develops after any kind of trauma, particularly limb fracture or soft tissue injury. Type I CRPS does not imply nerve damage.
In terms of magnitude, the symptoms exceed the expected clinical course after the initial incident, producing important and variably progressing motor impairment. The pain, which may be spontaneous or induced (allodynia or hyperalgesia), is always disproportionate to the initial event and does not follow the neurological trajectory of any nerve or root. It is accompanied by vasomotor and perspiration alterations, skin dystrophy and motor disorders — particularly enhanced physiological tremor. - Type II CRPS (previously referred to as causalgia). According to the new ICD-11, type II CRPS develops after trauma associated to peripheral nerve damage as evidenced by the neurological exploration, the electrodiagnostic tests or other quasi-objective tests. Although the clinical characteristics of the nerve lesion (numbness) are limited to the affected nervous territory, the signs and symptoms of CRPS must extend beyond the identified nervous territory. The signs and symptoms diagnosing type I and type II CRPS are identical.
Pathophysiology
The capacity to detect harmful stimuli is a protective mechanism found in most organisms, and is crucial for survival. There are two types of acute postsurgical pain: inflammatory and neuropathic. Inflammatory pain is the consequence of the release of local inflammatory mediators as a response to the painful stimulus. Neuropathic pain is produced as a result of damage to the nerves or sensory systems of the spinal cord and brain(7). An acute lesion, as observed in surgery or trauma, causing chronic pain, is associated to neuroplastic changes in the peripheral and central nervous system in response to the nociceptive input. These changes lead to hypersensitivity of the nervous system, which in turn promotes protection of the damaged area. The persistence of these changes often leads to weakening chronic pain. Thus, pain of the inflammatory kind becomes chronic when incomplete tissue repair occurs. Structural damage to a nerve is more likely to lead to chronic pain than pain secondary to damage to somatic tissue structures(24). Three inter-related processes may act as targets for the prevention of chronic pain, namely peripheral sensitisation, central sensitisation and descending modulation(25).
Risk factors
A number of risk factors are contemplated(26), as detailed below.
Preoperative
- Preoperative pain severity: In 2019, Edgley(27) found the greatest preoperative pain intensity in 326 trauma patients following surgery to be associated to chronic pain, which was observed in 65% of the cases.
- Psychological factors: negative preoperative thoughts, anxiety, depression, catastrophising.
- Genetic predisposition: for example, the alpha subunit of the potassium channels involved in neuron excitability. The holotype of the cyclooxygenase 1 gene is associated with less pain following discectomy due to persistent radicular lower back pain; it is also correlated to mRNA expression of the signal transduction genes(28).
- Female gender(27).
- Previous surgery(27).
- Advanced age (controversial).
Intraoperative
- Type of anaesthesia: risk of nerve damage in epidural, spinal or locoregional anaesthesias(29,30).
- Surgical technique: procedures involving cutting, contusion, compression, inflammation or thermal or ischaemic damage to a nerve. Tissue damage.
- Duration of surgery.
- Intraoperative pain.
Postoperative
- Severity and duration of postoperative pain: this is the most constant characteristic associated to the appearance of CPSP.
- Radiotherapy.
- Chemotherapy.
Chronic pain and types of surgery
Few publications are available on CPSP in orthopaedic surgery and trauma — the first studies having been focused on amputations (observed in 60% of the cases) and hip, knee and — in recent years — shoulder replacement surgery.
In general, surgeries on bone structures and joints show similar prevalences of CPSP, in the order of 20%(10).
Hip surgery
Hip prosthesis
In 1996, MacWilliam(31), in a series of 848 hip replacement surgeries, found factors such as patient race, education, number of comorbidities and the preoperative Health Status Questionnaire (HSQ) score to have an impact upon pain and physical function at 6 months postsurgery. Patients with poorer preoperative scores were seen to have poorer postoperative scores. For each 10-point increase in the preoperative scores, the patients could expect a decrease of at least 6 points in postoperative improvement.
Thus, preoperative status is an important predictor of hip replacement surgery outcome. It is known that in hip surgery, higher preoperative visual analogue scale (VAS) scores are associated to lesser postoperative improvement.
Autologous graft harvesting from the iliac crest
Bone graft harvesting from the iliac crest for surgery is associated to significant morbidity, including functional impairment and intense pain at the extraction site in approximately 3% of all patients(32,33).
Knee surgery
Localised CPSP with a neuropathic component has been seen in knee prostheses or arthroscopy of the knee in 11.4-44.4% of all cases(34).
Knee prosthesis
There is evidence that 30% of all patients with osteoarthrosis suffer central sensitisation symptoms(35,36).
In 2011, Hochman(37) suggested that 25% of all patients with knee osteoarthrosis present symptoms of neuropathic pain. Patients with intense pain for over 12 months have poorer postsurgical outcomes(38).
In 1999, Fortin(39), after studying 220 joint prostheses (including total hip and total knee replacements), suggested that patient baseline pain and function is the best predictor of pain and function 6 months after total hip or knee replacement surgery. In addition, advanced functional loss due to osteoarthrosis of the hip or knee was associated to poorer outcomes at 6 months. The patients with poorer preoperative function remained significantly worse after the operation. The poorer the preoperative functional status, the poorer the postoperative functional status.
Following total knee replacement surgery, the prevalence of chronic pain is 22-44%, with the pain being of neuropathic characteristics in up to 20% of the cases(40,41).
Recent cohort-based studies have yielded similar results, with 16-33% of all patients reporting chronic pain after total knee replacement surgery(41).
Arthroscopy of the knee
In 1998, Small(42) described four cases of reflex sympathetic dystrophy (type I CRPS) out of 10,262 arthroscopy procedures.
In 2008, Rosseland(43) estimated the prevalence of chronic pain after knee arthroscopy in both genders to be approximately 30%, with the pain being moderate to intense in 10% of the cases.
Shoulder surgery
In 2012, Desai et al.(44) concluded that preoperative pain and expected postoperative pain are predictors of actual postoperative pain.
Shoulder arthroplasty
The type of diagnosis, the type of prosthesis, previous shoulder surgeries, patient age and the Short Form-36 Health Survey (SF-36) and Disabilities of the Arm, Shoulder and Hand (DASH) scores have been regarded as predictors of the chronification of postsurgical pain(45).
A 22% prevalence of chronic pain beyond 1-2 years has been reported, with a 4-13% prevalence of neuropathic pain following shoulder arthroplasty. In turn, chronic pain is more frequent following fracture (29%) than in the context of osteoarthrosis (16%), while the prevalence of neuropathic pain is estimated to be similar(46,47).
Increased patient sensitivity to preoperative pain (lower pain threshold and greater pain under resting conditions) implies poorer functional recovery and greater postsurgical pain(48).
Rotator cuff rupture
In 2019, De Boer reported that patients subjected to rotator cuff repair suffer more pain than patients undergoing other types of surgeries in that anatomical region, and even pointed to the female gender and subacromial decompression with distal resection of the clavicle as significant risk factors for intense postsurgical pain. This author therefore postulated that different protocols are needed according to the type of patient and the type of surgery(49).
Preoperative pain control is of clinical relevance in patients subjected to arthroscopic rotator cuff repair(50).
In a cohort study published in 2021, Rizvi(51) concluded that the magnitude and frequency of preoperative pain are the most important factors conditioning postoperative pain after arthroscopic surgery for rotator cuff rupture.
A total of 15.8% of all patients with complete rupture of the rotator cuff suffer neuropathic pain — the latter being related to the pain VAS score of the last four weeks and increased degree cuff rupture(52).
Prevention
The evidence on prevention is still limited: the long-term benefit of preventive-perioperative analgesia has not been consistently demonstrated(8).
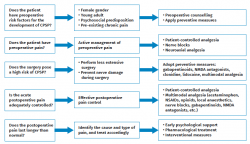
Prevention is applied at three levels (Figure 1): preoperative, perioperative and postoperative(53).
Preoperative actions
- Preoperative pain control: Karanikolas et al.(54) used optimised perioperative epidural analgesia in 65 patients subjected to lower limb amputation. Patient-controlled analgesia (PCA) was started 48 hours before the operation, and continued for another 48 hours after the operation, compared with the patients that received conventional analgesia and general anaesthesia. The incidence of phantom limb pain after 6 months decreased significantly.
- Reduce inflammation.
- Improve the blood count.
- Control overweight.
- Increase muscle tone.
- Control anxiety.
Intraoperative actions
- Control preventive ischaemia.
- Neuroaxial analgesia — peripheral nerve blocks. The use of preventive analgesia (which affords pain control throughout the perioperative phase, thus blocking the harmful stimulus during this painful period) instead of intraoperative analgesia has also shown benefits in the prevention of CPSP(55,56).
A study compared three analgesic techniques following thoracotomy: thoracic epidural analgesia started pre- and intraoperatively (with patient-controlled epidural analgesia administered preoperatively in both cases) and patient-controlled intravenous analgesia with morphine, started after surgery. Chronic postsurgical pain decreased significantly 6 months after the operation with the use of thoracic epidural analgesia started preoperatively(57). - Correct incision. Extensive approaches should be avoided as far as possible, minimising tissue damage. Nevertheless, arthroscopic surgeries are still able to cause CPSP as a result of nerve damage (e.g., branches of the saphenous nerve during knee arthroscopy), since it is not always possible to avoid such damage, due to the proximity of the nerves to the access route or bone structure.
- Preservation of soft tissues, precise surgical approach.
- Avoidance of excessive electrical scalpel use.
- Saws in good condition.
- Avoidance of excessive distraction of the different tissue levels.
- Intraoperative infiltration: the intraarticular injection of local anaesthetics during arthroplasty and joint surgeries may be effective in securing improved postoperative pain control. Wound infiltration with local anaesthetics following iliac crest bone graft harvesting has been reported to decrease chronic iliac bone pain over four years of follow-up(33). Even so, wound infiltration affords only brief pain relief, without influencing the risk of developing chronic pain(58).
- Surgeon experience: centres with surgeons undergoing training usually have higher chronic pain rates(59).
- Duration of surgery: operations that last over three hours are associated to increased CPSP(55,57).
- Control of intraoperative acute pain: neuroplasticity (spinal sensitisation) after trauma may transform acute pain into chronic pain if not adequately dealt with. This situation can be prevented through exhaustive treatment of the acute pain(33).
The use of intravenous lidocaine and perioperative EMLA cream has been found to reduce the incidence of chronic pain following mastectomy, with no significant impact in cases of acute pain(58).
Karanikolas(54) recorded no difference in the incidence of chronic phantom limb pain following amputation with epidural analgesia during the perioperative period, compared with the use of patient-controlled opioid analgesia during that same period. Therefore, good pain control during the perioperative period seems more important than the technique used to achieve pain control.
The best strategy is multimodal analgesia during the perioperative period for the management of acute postoperative pain(60). By acting via different mechanisms, the drugs can control pain more effectively than when a single drug is used, since they modulate the pain signals at various points over the nociceptive pathway. Multimodal pain treatments can include combinations of gabapentin, non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen and regional anaesthesia with the conventional analgesic technique(60). Very few studies have been carried out to date in traumatology on the effects of multimodal analgesia upon CPSP.
Postoperative actions
- Control of acute postoperative pain: the intensity and persistence of acute postoperative pain is the strongest predictor of the appearance of chronic pain(29,61,62).
The persistence of postoperative pain after weeks 1-6 is related to the presence of CPSP three months after the operation, though the incidence gradually decreased from 6 months to one year postsurgery. All this is related to peripheral sensitisation and central sensitisation. - Early mobilisation (< 24 hours).
- Detection of complications.
Psychological intervention
Depression, psychological vulnerability, stress and a late return to work are risk factors for CPSP(63). Patient catastrophising of pain is sometimes related to a decrease in CPSP, perhaps because of the early search for medical help12: Patients with negative conceptions about opioids have a greater risk of suffering CPSP(60).
Adequate preoperative information, as well as discussion of the individual expectations, may alleviate stress and help prevent CPSP. Thus, intervention before and after surgery and the screening of psychologically vulnerable patients may help prevent chronic pain(55).
Preventive drug treatment
Different treatments have been evaluated for preventing chronic postsurgical neuropathic pain. A Cochrane review(64) in 2013 reported on the evidence in the treatment of CPSP and concluded that: "Additional proof is needed from better, well designed large-scale trials to ensure more rigorous evaluation of the drug interventions for the prevention of chronic pain after surgery. In addition, the available evidence does not support the efficacy of gabapentin, pregabalin, non-steroidal anti-inflammatory drugs, intravenous corticosteroids, oral N-methyl-D-aspartate (NMDA) blockers, oral mexiletine, intravenous fentanyl, intravenous lidocaine, oral venlafaxine or inhaled nitrous oxide for the prevention of postoperative pain".
Subsequent studies have positioned the use of the different drugs.
Gabapentin and pregabalin
Mishriky(65) published a systematic review and meta-analysis in which perioperative pregabalin was associated to a significant reduction of opioid use after surgery, and to a significant decrease in the incidence of pain at 6 and 12 months.
The result of gabapentin and pregabalin is considered to be due to the preventive analgesic effect afforded by the gabapentinoids(66). However, a recent review of 18 randomised controlled trials with published and non-published studies concluded (with a moderate level of evidence) that pregabalin is unable to reduce the incidence of CPSP at three months, though it may influence the incidence of CPSP with a neuropathic component(67).
Antidepressants
Because of the heterogeneity of the published studies, no conclusions can be drawn regarding the capacity of antidepressants to prevent CPSP. Wong(68) examined three trials involving the use of venlafaxine, duloxetine and escitalopram, and only documented positive outcomes with venlafaxine. In 2019, Koh(69) concluded that perioperative duloxetine could improve the quality of recovery and reduce the development of CPSP in patients with preoperative central sensitisation.
N-methyl-D-aspartate (NMDA) receptor antagonists
Different studies have demonstrated the usefulness of a perioperative subanaesthetic dose of ketamine for preventing phantom limb pain(25).
Ketamine appears to be the drug offering the most consistent positive results in terms of preventive analgesia(60). The perioperative use of intravenous ketamine has been shown to be useful in terms of prevention, particularly in highly painful procedures such as orthopaedic surgery(70).
The use of other NMDA antagonists such as memantine has not been established to date, though pain relief does not seem to be maintained long enough to prevent CPSP(25,55).
Alpha-2 agonists
An example of such drugs is clonidine. Few studies have focused on the prevention of CPSP, though this drug could play a role in view of its anti-inflammatory and anti-sensitising effects(71).
Lidocaine
Lidocaine is used as a local aesthetic in perineural injections, and its intravenous administration for the management of chronic pain is also increasing, as well as perioperative use for reducing acute postoperative pain. In 2013, a Cochrane review(64) was unable to draw conclusions on the effects of lidocaine in this regard. Posteriorly, in 2018, a new review concluded that continuous wound infiltration can reduce CPSP in the context of bone graft harvesting from the iliac crest(72). However, further studies are needed before its use can be recommended for the prevention of CPSP in other areas.
Non-steroidal anti-inflammatory drugs (NSAIDs)
Although NSAIDs offer beneficial effects in relation to acute pain, reduce the need for opioids, and are believed to reduce secondary hyperalgesia and central sensitisation, their effects upon CPSP have not been demonstrated in any of the multiple studies published to date. The use of ibuprofen for the prevention of chronic pain following hip replacement surgery has not shown any significant decrease in the incidence of CPSP(73).
The role of acetaminophen has not been clarified to date, since no randomised controlled trials have been made to assess its effect in preventing CPSP — though its use currently forms part of the integral multimodal management of perioperative pain.
Reuben(74) studied 200 patients subjected to anterior cruciate ligament surgery administering acetaminophen (1 g) and celecoxib versus placebo during 1-2 hours before the operation together with intraarticular analgesics, and found the control subjects to develop more femoropatellar complications, including anterior knee pain and CRPS, among others, 6 months after surgery. Later, however (in 2009) this study was shown to include fraudulent data.
Corticosteroids
In view of their anti-inflammatory effects, and knowing that the development of chronic pain implies neuroinflammation, corticosteroids could prove promising for the prevention of CPSP. In 2009, Bergeron recorded no differences in CPSP on administering 40 mg of dexamethasone before total hip arthroplasty(75). A randomised, prospective study involving one year of follow-up evaluated the use of 16 mg of intravenous dexamethasone after lumbar discectomy, and documented a significantly higher pain score in the dexamethasone group — though there were no differences in patient-reported work capacity, disability or health(76). A recent meta-analysis published by Zhu(77) has estimated that the intravenous administration of a single dose of dexamethasone before total knee replacement surgery is probably effective and safe in reducing postoperative pain, opiate consumption and the risk of nausea / vomiting after surgery.
Opioids
Opioids are the analgesics of choice for intra- and postoperative analgesia in situations characterised by moderate to intense pain, and since intense postoperative is a risk factor for CPSP, these drugs may contribute to prevent the latter. Good pain control with opioids is important for the prevention of CPSP, despite the known risk of hyperalgesia (remifentanil)(54).
Other drugs
Few studies can be found on the use of dextromethorphan, mexiletine and nitrous oxide, and the reported effects upon CPSP are moreover variable(64). It is therefore not possible to recommend the use of these drugs for the prevention of CPSP.
Diagnosis and differential diagnosis
As always, a detailed case history and physical examination are very important for defining the type of pain. A differential diagnosis must be established with the pain present during the preoperative period, the postoperative complications (including particularly infections), or recurrence of the primary disease condition.
Over 50% of all patients with CPSP suffer neuropathic pain, and the rest present nociceptive (somatic or visceral) pain. All the pain components must be detailed in order to afford correct treatment. During the preoperative and early postoperative period, the patient must be informed about the possibility of developing CPSP. Those patients who develop CPSP, and their relatives, are to be informed about the prognosis, management plan and rehabilitation. The patients in turn are to be implicated in the strategies for self-care and return to normal activities of daily living.
Treatment
Non-pharmacological treatment
Changes in lifestyle
Physical therapies (massages, physiotherapy and acupuncture) can lessen the pain, though only temporarily. All such treatments are to be applied within the context of no pain or controlled pain.
Rest or the limitation of activity is not advised, since it may result in poorer functional outcomes(53).
Psychological therapy
Both behavioural therapy (based on reinforcement principles, focusing on modifying responses to maladaptive behaviours and — in the case of chronic pain — behaviours consisting of open expressions of pain, distress and suffering)(78) and cognitive-behavioural therapy (not focused on eliminating pain but on improving physical and emotional function despite the pain)(79) are useful for the management of CPSP(80,81).
In 2006, Brox(82) carried out a randomised study of patients with CPSP following disc hernia surgery, comparing the effectiveness of lumbar fusion with subsequent transpedicular screw fixation versus cognitive intervention and exercises. Surgery versus combined cognitive intervention and exercise showed similar effectiveness in the management of pain. In 2002, Cohen(83) reported that intense exercise associated to cognitive-behavioural therapy is useful for dealing with back pain of different origins, including back pain manifesting after surgery.
Pharmacological treatment
Few studies can be found on the use of drugs to treat CPSP without a neuropathic component, and most of the recommendations have been extrapolated from data corresponding to other types of chronic pain, particularly neuropathic pain.
Thus, use is made of anticonvulsants (gabapentin and pregabalin), tricyclic antidepressants (amitriptyline and nortriptyline), serotonin — noradrenaline reuptake inhibitors (duloxetine and venlafaxine), topical lidocaine or first line treatment with topical capsaicin.
Paracetamol, NSAIDs and weak opioids (tramadol and codeine) can be used in accordance with the severity of the symptoms.
Potent opioids are to be used with caution, duly assessing the risks and benefits. Other drugs that may be useful are ketamine, muscle relaxants, clonidine and intravenous lidocaine infusion.
Based on a retrospective analysis of case reports, a meeting of 44 pain specialists from 17 countries concluded that CPSP associated to localised and superficial pain, and allodynia, showed a positive response to 5% lidocaine dressings(84).
An observational study of patients with posttraumatic and postoperative localised neuropathic pain (LNP) also revealed a significant decrease in pain and of the painful areas following treatment in the form of lidocaine patches(85,86).
The use of topical 1% amitriptyline associated to 0.5% ketamine for the treatment of neuropathic pain — including CPSP — appears promising(87).
An open-label prospective study involving a 2% amitriptyline and 1% ketamine combination cream applied during 6-12 months evidenced significant pain relief, with long-term patient satisfaction and minimal side effects(88).
Interventional treatment
Nerve blocks, neuroaxial blocks and sympathectomies have been considered.
Pain relief has been observed with epidural injections for lumbar or cervical postoperative syndrome(89), though effectiveness was only recorded with combined epidural corticosteroid and local anaesthetic injections — not with isolated individual drug injections(90,91).
Axillary brachial plexus block with patient-controlled analgesia is useful for treating type I CRPS manifesting after surgical release in patients with carpal tunnel syndrome(92).
Phenol injections or radiofrequency ablation in the neuroma and dorsal root ganglia are useful in application to stump pain and phantom limb pain(93,94).
When other options fail, coagulation of the dorsal root entry zone (DREZ) and motor cord stimulation have been shown to be useful. Radiofrequency based percutaneous partial rhizotomy, and pulsed radiofrequency applied to the ganglia of the dorsal root with those of the intercostal nerve also prove useful. Ablation and pulsed radiofrequency, transcutaneous electrical nerve stimulation and mirror therapy can offer benefits for patients with phantom limb syndrome(95).
Spinal cord stimulation has shown promising results in application to phantom limb pain(96). It is also effective for the treatment of neuropathic pain of the upper and lower extremities following neck or spinal surgery, and in type I CRPS(17).
Surgical treatment
No relevant information is available in orthopaedic surgery or traumatology. Based on publications corresponding to other surgical fields, surgical resection of the neuroma and retraction of the nerve in muscle planes appears to be useful in post-mastectomy patients(97). Repositioning the nerve in a protected location and helping it to grow by means of a graft may also be useful(94,95).
Likewise, an autologous adipose tissue graft at the dermal-hypodermal interface in the area of the painful scar proves useful(98).
Scar resection in inguinal hernia may also help to alleviate pain after surgery, in the same way as the removal of material (mesh)(99).
The recurrence of CPSP after such surgeries cannot be ruled out; therefore, the adoption of preventive measures is crucial, including effective perioperative information and treatment of the pain. These references based on other areas in which nerve damage occurs may serve as a guide and encourage the conduction of specific studies in traumatology.
Multidisciplinary teams are required to adequately address the complex process of chronification of postsurgical pain with a high incidence of neuropathic pain, including not only biological factors but also psychosocial aspects(100,101).
Conclusions
It can be concluded that large scale prospective studies with detailed multifactorial pre-, intra- and postoperative evaluations are needed to improve our understanding of the aetiology and prognosis of CPSP(8).
At present, given the limited knowledge regarding effective treatment, interventions targeted to the risk factors could improve the incidence of CPSP, as described by Thapa(53) in 2018. Individualised prevention in patients at high risk of suffering chronification could increase the success of the preventive measures.
In relation to the adequate evaluation and management of these risk factors, multidisciplinary teams are required to characterise the experience of postoperative pain in each individual patient and examine the pain that manifests during his or her functional activity. On the other hand, it is important to assess the magnitude of the inflammatory component and the neuropathic component of CPSP in order to guide multimodal treatment.
Información del artículo
Cita bibliográfica
Autores
Luis Javier Roca Ruíz
Unidad de Hombro. Servicio de Cirugía Ortopédica y Traumatología. Hospital Universitario Virgen Macarena. Sevilla
Ethical responsibilities
Conflicts of interest. The authors state that they have no conflicts of interest.
Financial support. This study has received funding from Grünenthal.
Protection of people and animals. The authors declare that this research has not involved human or animal experimentation.
Data confidentiality. The authors declare that the protocols of their work centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Margarit C. La nueva clasificación internacional de enfermedades L (CIE-11) y el dolor crónico. Implicaciones prácticas. Rev Soc Esp Dolor. 2019;209-10.
-
2Macrae W, Davis HTO. Chronic Post-surgical Pain. En: Crombie IK, Linton S, Croft P, Von Korff M, LeResche L (eds.). Epidemiology of pain. Seattle: IASP Press; 1999. pp. 125-42.
-
3Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87(1):88-98.
-
4Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-46.
-
5Werner MU, Kongsgaard UE. Defining persistent post-surgical pain: Is an update required? Br J Anaesth. 2014;113(1):1-4.
-
6The Lancet. Icd-11. Lancet. 2019;393(10188):2275.
-
7Ribera H, Esteve N, Garrido JP. La transición de dolor agudo postoperatorio a crónico: ¿Qué sabemos? Rev Soc Esp Dolor. 2012;19(4):197-208.
-
8Bruce J, Quinlan J. Chronic post surgical pain 2010-Searle-12-4. Rev Pain. 2011;5(3):23-9.
-
9Torres LM, Gálvez R. Dolor neuropático periférico localizado. Asociación Andaluza del Dolor; 2017.
-
10Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95-102.
-
11Crombie IK, Davies HTO, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76(1-2):167-71.
-
12Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective fa. Expert Rev Neurother. 2009;9(5):723-44.
-
13Schug SA, Pogatzki-Zahn EM. Chronic pain after surgery or injury. International association for the study of pain. Pain. 2011;19(1):19.
-
14Ong CKS, Seymour RA. Pathogenesis of Postoperative Oral Surgical Pain. Anesth Prog. 2003;50(1):5-17.
-
15Schecter WP, Bongard FS, Gainor BJ, Weltz DL, Horn JK, General SF. Pain Control in Outpatient Surgery. J Am Coll Surg. 2002 Jul;195(1):95-104.
-
16Fletcher D, Stamer UM, Pogatzki-Zahn E, et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32(10):725-34.
-
17Shipton E. Post-surgical neuropathic pain. ANZ J Surg. 2008;78(7):548-55.
-
18Macrae WA. Chronic post-surgical pain: 10 Years on. Br J Anaesth. 2008;101(1):77-86.
-
19Montaner MC, Soler E, Faus MT. El dolor postoperatorio en la actualidad: un problema de calidad asistencial. Farm Hosp. 2000;24(3):118-75.
-
20Mick G, Baron R, Finnerup NB, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manag. 2012 Jan;2(1):71-7.
-
21Lara-Solares A, Mayoral-Rojals V, Guillén-Núñez MR, et al. Consenso multidisciplinario de diagnóstico y tratamiento del dolor neuropático periférico y localizado en México. Gac Med Mex. 2019;155(4):428-35.
-
22Martyn CN, Hughes RAC. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(4):310-8.
-
23Plancarte-Sánchez R, Samano-García M, Guillén-Núñez M del R, Equihua-Ortega A. Dolor neuropático localizado. Gac Med Mex. 2021;157(3):9-11.
-
24Velasco M. Dolor neuropático. Rev Med Clin Condes. 2014;25(4):625-34.
-
25McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl. 2011;5(2):365-76.
-
26Althaus A, Hinrichs-Rocker A, Chapman R, et al. Development of a risk index for the prediction of chronic post-surgical pain. Eur J Pain. 2012;16(6):901-10.
-
27Edgley C, Hogg M, de Silva A, Braat S, Bucknill A, Leslie K. Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: a cohort study. Br J Anaesth. 2019;123(3):350-9.
-
28Saxena AK, Chilkoti GT, Chopra AK, Banerjee BD, Sharma T. Chronic persistent post-surgical pain following staging laparotomy for carcinoma of ovary and its relationship to signal transduction genes. Korean J Pain. 2016;29(4):239-48.
-
29Perkins FM, Kehlet H. Chronic Pain as an Outcome of Surgery. Anesthesiology. 2000;93:1123-33.
-
30Lenters TR, Davies J, Matsen FA. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-87.
-
31MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-38.
-
32Heary RF, Schlenk RP, Sacchieri TA, et al. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50(3):510-7.
-
33Kraychete DC, Sakata RK, Lannes L de OC, Bandeira ID, Sadatsune EJ. Postoperative persistent chronic pain: what do we know about prevention, risk factors, and treatment. Braz J Anesthesiol. 2016;66(5):505-12.
-
34Pickering G, Martin E, Tiberghien F, Delorme C, Mick G. Localized neuropathic pain: an expert consensus on local treatments. Drug Des Devel Ther. 2017;11:2709-18.
-
35Akinci A, al Shaker M, Chang MH, et al. Predictive factors and clinical biomarkers for treatment in patients with chronic pain caused by osteoarthritis with a central sensitisation component. Int J Clin Pract. 2016;70(1):31-44.
-
36Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573-81.
-
37Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19(6):647-54.
-
38Jones CA, Voaklander DC, Johnston WC, Suarez-Almazor ME. The effect of age on pain, function, and quality of life after total hip and knee arthroplasty. Arch Intern Med. 2001;161(3):454-60.
-
39Fortin PR, Clarke AE, Joseph L, et al. Outcomes of total hip and knee replacement: Preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42(8):1722-8.
-
40Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566-72.
-
41Wylde V, Beswick A, Bruce J, Blom A, Howells N, Gooberman-Hill R. Chronic pain after total knee arthroplasty. EFORT Open Rev. 2018 Aug;3(8):461-70.
-
42Small NC. Complications in arthroscopic surgery performed by experienced arthroscopists. Arthroscopy. 1988;4(3):215-21.
-
43Rosseland LA, Solheim N, Stubhaug A. Pain and disability 1 year after knee arthroscopic procedures. Acta Anaesthesiol Scand. 2008;52(3):332-7.
-
44Desai VN, Cheung E. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-50.
-
45Simmen BR, Bachmann LM, Drerup S, Schwyzer HK, Burkhart A, Goldhahn J. Development of a predictive model for estimating the probability of treatment success one year after total shoulder replacement - cohort study. Osteoarthritis Cartilage. 2008;16(5):631-4.
-
46Razmjou H, Woodhouse LJ, Holtby R. Neuropathic pain after shoulder arthroplasty: prevalence, impact on physical and mental function, and demographic determinants. Physiother Can. 2018;70(3):212-20.
-
47Bjørnholdt KT, Brandsborg B, Søballe K, Nikolajsen L. Persistent pain is common 1-2 years after shoulder replacement: a nationwide registry-based questionnaire study of 538 patients. Acta Orthop. 2015;86(1):71-7.
-
48Kadum B, Inngul C, Ihrman R, Sjödén GO, Sayed-Noor AS. Higher preoperative sensitivity to pain and pain at rest are associated with worse functional outcome after stemless total shoulder arthroplasty: a prospective cohort study. Bone Joint J. 2018;100B(4):480-4.
-
49De Boer FA, Schouten TTJ, Boekestein EP, et al. Risk factors for postoperative pain in the first three weeks after arthroscopic or open shoulder surgery. Orthop Traumatol Surg Res. 2019;105(2):241-4.
-
50Tonotsuka H, Sugaya H, Takahashi N, Kawai N, Sugiyama H, Marumo K. Preoperative pain control in arthroscopic rotator cuff repair: Does it matter? CiOS Clinics in Orthopedic Surgery. 2019;11(2):192-9.
-
51Rizvi SMT, Bishop M, Lam PH, Murrell GAC. Factors Predicting Frequency and Severity of Postoperative Pain After Arthroscopic Rotator Cuff Repair Surgery. Am J Sports Med. 2021;49(1):146-53.
-
52Ko S, Choi C, Kim S, Chae S, Choi W, Kwon J. Prevalence and risk factors of neuropathic pain in patients with a rotator cuff tear. Pain Physician. 2018;21(2):E173-80.
-
53Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 2018;31(3):155-73.
-
54Karanikolas M, Aretha D, Tsolakis I, Monantera G, Swarm RA, Filos KS. Optimized Perioperative Analgesia Reduces Chronic. Anesthesiology. 2011;114(5):1144-54.
-
55Reddi D. Preventing chronic postoperative pain. Anaesthesia. 2016;71:64-71.
-
56Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesthesia Analgesia. 2012;115(2):428-42.
-
57Şentürk M, Özcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesthesia Analgesia. 2002;94(1):11-5.
-
58Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain. 2015;19(4):451-65.
-
59Tasmuth T, Blomqvist C, Kalso E. Chronic post-treatment symptoms in patients with breast cancer operated in different surgical units. Eur J Surg Oncol. 1999;25(1):38-43.
-
60Clarke H, Poon M, Weinrib A, Katznelson R, Wentlandt K, Katz J. Preventive analgesia and novel strategies for the prevention of chronic post-surgical pain. Drugs. 2015;75(4):339-51.
-
61Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5(1):89-96.
-
62Poleshuck EL, Katz J, Andrus CH, et al. Risk Factors for Chronic Pain Following Breast Cancer Surgery: A Prospective Study. J Pain. 2006;7(9):626-34.
-
63Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EAM. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - A systematic review. Eur J Pain. 2009;13(7):719-30.
-
64Chaparro LE, Smith S, Moore RA, Gilron I. Pharmacotherapy for the Prevention of Chronic Pain after Surgery in Adults: review. Anesthesiology. 2021;(7):304-25.
-
65Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10-31.
-
66Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119(5):1215-21.
-
67Martinez V, Pichard X, Fletcher D. Perioperative pregabalin administration does not prevent chronic postoperative pain: systematic review with a meta-analysis of randomized trials. Pain. 2017;158(5):775-83.
-
68Wong K, Phelan R, Kalso E, et al. Antidepressant drugs for prevention of acute and chronic postsurgical pain early evidence and recommended future directions. Anesthesiology. 2014;121(3):591-608.
-
69Koh I, Kim M, Sohn S, Song K, Choi N, In Y. Duloxetine Reduces Pain and Improves Quality of Recovery Following Total Knee Arthroplasty in Centrally Sensitized Patients: A Prospective, Randomized Controlled Study. J Bone Joint Surg Am. 2019;101(1):64-73.
-
70McNicol ED, Schumann R, Haroutounian S. A systematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain. Acta Anaesthesiol Scand. 2014;58(10):1199-213.
-
71Van de Ven TJ, John Hsia HL. Causes and prevention of chronic postsurgical pain. Curr Opin Crit Care. 2012;18(4):366-71.
-
72Weinstein EJ, Levene JL, Cohen MS, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. 2018;2018(4).
-
73Fransen M, Anderson C, Douglas J, et al. Safety and efficacy of routine postoperative ibuprofen for pain and disability related to ectopic bone formation after hip replacement surgery (HIPAID): randomised controlled trial. Br Med J. 2006;333(7567):519-21.
-
74Reuben SS, Ekman EF. The effect of initiating a preventive multimodal analgesic regimen on long-term patient outcomes for outpatient anterior cruciate ligament reconstruction surgery (Retraction in: Anesth Analg. 2009 Apr;108(4):1350). Anesth Analg. 2007;105(1):228-32.
-
75Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J. Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res. 2009;467(6):1463-7.
-
76Nielsen RV, Fomsgaard J, Mathiesen O, Dahl JB. The effect of preoperative dexamethasone on pain 1 year after lumbar disc surgery: a follow-up study. BMC Anesthesiol. 2016;16(1):1-9.
-
77Zhu M, Chang Y, Phillips S, Scholey A, Bhandari M. Preoperative Single-Dose Intravenous Dexamethasone for Total Knee Arthroplasty: A Systematic Review and Meta- Analysis. Orthoevidence. 2022;5(3).
-
78Patterson DR. Behavioral methods for chronic pain and illness: a reconsideration and appreciation. Rehabil Psychol. 2005;50(3):312-5.
-
79Turk DC, Meichenbaum D, Genest M. Pain and Behavioral Medicine: A Cognitive-Behavioral Perspective. New YorK, NY: Guilford; 1983.
-
80Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-52.
-
81Turk DC, Audette J, Levy RM, Mackey SC, Stanos S. Assessment and treatment of psychosocial comorbidities in patients with neuropathic pain. Mayo Clin Proc. 2010;85(3 Suppl.):S42-50.
-
82Brox JI, Reikerås O, Nygaard Ø, et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: A prospective randomized controlled study. Pain. 2006;122(1-2):145-55.
-
83Cohen I, Rainville J. Aggressive exercise as treatment for chronic low back pain. Sports Med. 2002;32(1):75-82.
-
84Nicolaou A, Nicholson B, Hans G, Brasseur L. Outcome predictors for treatment success with 5% lidocaine medicated plaster in low back pain with neuropathic components and neuropathic pain after surgical and nonsurgical trauma. J Pain Res. 2011;4:25-38.
-
85Correa-Illanes G, Roa R, Piñeros JL, Calderón W. Use of 5% lidocaine medicated plaster to treat localized neuropathic pain secondary to traumatic injury of peripheral nerves. Local Reg Anesth. 2012;5(1):47-53.
-
86Hans G, Joukes E, Verhulst J, Vercauteren M. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Curr Med Res Opin. 2009;25(11):2737-43.
-
87Lynch ME, Clark AJ, Sawynok J. A pilot study examining topical amitriptyline, ketamine, and a combination of both in the treatment of neuropathic pain. Clin J Pain. 2003;19(5):323-8.
-
88Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neuropathic pain syndromes: an open-label study. J Pain. 2005;6(10):644-9.
-
89Abdi S, Datta S, Trescot AM, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;10(1):185-212.
-
90Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Management of pain of post lumbar surgery syndrome: one-year results of a randomized, double-blind, active controlled trial of fluoroscopic caudal epidural injections. Pain Physician. 2010;13(6):509-21.
-
91Manchikanti L, Malla Y, Cash KA, Mcmanus CD, Pampati V. Fluoroscopic cervical interlaminar epidural injections in managing chronic pain of cervical postsurgery syndrome: preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012;15(1):13-26.
-
92Wang LK, Chen HP, Chang PJ, Kang FC, Tsai YC. Axillary brachial plexus, block with patient controlled analgesia for complex regional pain syndrome type I: a case report. Reg Anesth Pain Med. 2001;26(1):68-71.
-
93Ganapathy S, Brookes J. Chronic postsurgical pain after nonarthroplasty orthopedic surgery. Tech Reg Anesth Pain Manag. 2011;15(3):116-23.
-
94Ramanavarapu V, Simopoulos TT. Pulsed radiofrequency of lumbar dorsal root ganglia for chronic post-amputation stump pain. Pain Physician. 2008;11(4):561-6.
-
95Subedi B, Grossberg GT. Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat. 2011;2011:864605.
-
96Manchikanti L, Singh V. Managing phantom pain. Pain Physician. 2004;7(3):365-75.
-
97Wong L, Bartlett SB. Intercostal neuromas: a treatable cause of postoperative breast surgery pain. Ann Plast Surg. 2001;46(5):481-4.
-
98Caviggioli F, Maione L, Forcellini D, Klinger F, Klinger M. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg. 2011;128(2):349-52.
-
99Hakeem A. Current trends in the diagnosis and management of post-herniorraphy chronic groin pain. World J Gastrointest Surg. 2011;3(6):73.
-
100Clarke H, Woodhouse LJ, Kennedy D, Stratford P, Katz J. Strategies aimed at preventing chronic post-surgical pain: Comprehensive perioperative pain management after total joint replacement surgery. Physiother Can. 2011;63(3):289-304.
-
101Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care. 2013;7(2):144-52.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- The importance of pain control
- Learning from pain to make the patient feel better
- Perioperative pain management in arthroscopic surgery of the shoulder
- Perioperative pain management in arthroscopy of the elbow
- Perioperative analgesia in arthroscopic surgery of the wrist and hand
- Perioperative analgesia in arthroscopy of the hip
- Perioperative analgesia in arthroscopic surgery of the knee
- Perioperative pain management in arthroscopy of the ankle
- Postoperative neuropathic pain in traumatology
- Capsaicin 179 mg patch application technique
- Type 3 SLAP: bucket handle tear
Más en PUBMED
Más en Google Scholar


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.